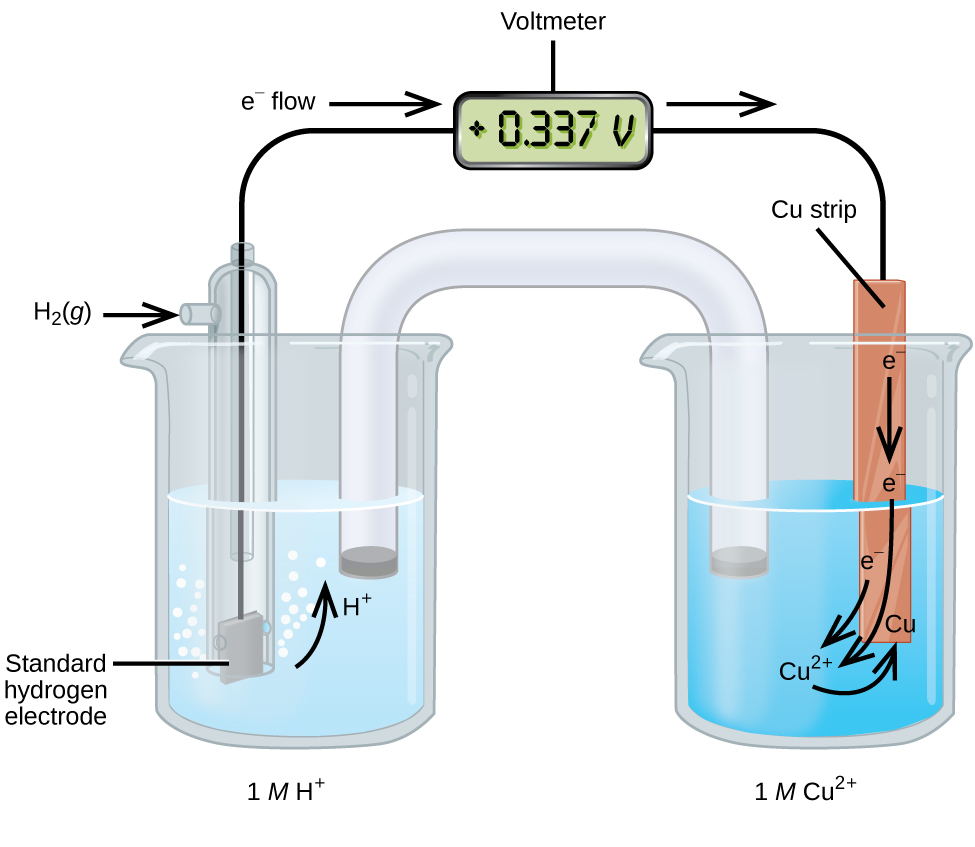
Standard Cell Potential And Standard Electrode Potential . The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. describe and relate the definitions of electrode and cell potentials. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative. by the end of this section, you will be able to: standard cell potential. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and.
from boisestate.pressbooks.pub
by the end of this section, you will be able to: Describe and relate the definitions of electrode and cell potentials. describe and relate the definitions of electrode and cell potentials. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. Interpret electrode potentials in terms of relative. standard cell potential. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound.
17.3 Standard Reduction Potentials General Chemistry 1 & 2
Standard Cell Potential And Standard Electrode Potential Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative. Describe and relate the definitions of electrode and cell potentials. standard cell potential. describe and relate the definitions of electrode and cell potentials. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. by the end of this section, you will be able to: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II Standard Cell Potential And Standard Electrode Potential Describe and relate the definitions of electrode and cell potentials. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. by the end of this section,. Standard Cell Potential And Standard Electrode Potential.
From meteonmendez.blogspot.com
Standard Electrode Potential Table MeteonMendez Standard Cell Potential And Standard Electrode Potential by the end of this section, you will be able to: in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. . Standard Cell Potential And Standard Electrode Potential.
From www.youtube.com
19.1 Calculate cell potentials using standard electrode potentials [HL Standard Cell Potential And Standard Electrode Potential by the end of this section, you will be able to: Interpret electrode potentials in terms of relative. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the. Standard Cell Potential And Standard Electrode Potential.
From boisestate.pressbooks.pub
17.3 Standard Reduction Potentials General Chemistry 1 & 2 Standard Cell Potential And Standard Electrode Potential in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Interpret electrode potentials in terms of relative. standard cell potential. describe and relate the definitions of electrode and cell potentials. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. Describe and relate. Standard Cell Potential And Standard Electrode Potential.
From exoeelsdz.blob.core.windows.net
Standard Electrode Potentials Chemguide at Richard Reddish blog Standard Cell Potential And Standard Electrode Potential in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. Describe and relate the definitions of electrode and cell potentials. by the end of this section, you will be able to: describe and. Standard Cell Potential And Standard Electrode Potential.
From exohmihef.blob.core.windows.net
Standard Electrode Potential Meaning at Francis McQuay blog Standard Cell Potential And Standard Electrode Potential The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. Describe and relate the definitions of electrode and cell potentials. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. in electrochemistry, standard electrode potential , or , is. Standard Cell Potential And Standard Electrode Potential.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Cell Potential And Standard Electrode Potential Interpret electrode potentials in terms of relative. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. by the end of this section, you will be able to: Describe and relate the definitions of electrode and cell potentials. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of. Standard Cell Potential And Standard Electrode Potential.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Cell Potential And Standard Electrode Potential Interpret electrode potentials in terms of relative. by the end of this section, you will be able to: standard cell potential. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Describe and relate the definitions of electrode and cell potentials. revision notes on 5.3.3 standard electrode & cell potentials. Standard Cell Potential And Standard Electrode Potential.
From thechemistrynotes.com
Cell Potential & Standard Cell Potential Standard Cell Potential And Standard Electrode Potential Describe and relate the definitions of electrode and cell potentials. standard cell potential. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. Interpret electrode potentials in terms of relative. by the end of this section, you will be able to: 42 rows calculate the standard cell potential of a voltaic cell that. Standard Cell Potential And Standard Electrode Potential.
From www.chegg.com
Solved Calculate the standard cell potential of the Standard Cell Potential And Standard Electrode Potential describe and relate the definitions of electrode and cell potentials. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. by the end of this section, you will be able to: Describe and relate the definitions of electrode and cell potentials. revision notes on 5.3.3 standard. Standard Cell Potential And Standard Electrode Potential.
From mungfali.com
Electrode Potential Series Standard Cell Potential And Standard Electrode Potential standard cell potential. by the end of this section, you will be able to: Interpret electrode potentials in terms of relative. describe and relate the definitions of electrode and cell potentials. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. 42 rows calculate the. Standard Cell Potential And Standard Electrode Potential.
From solvedlib.com
Using the following data, determine the standard cell… SolvedLib Standard Cell Potential And Standard Electrode Potential The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. standard cell potential. describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. by the end of this section,. Standard Cell Potential And Standard Electrode Potential.
From users.highland.edu
Standard Potentials Standard Cell Potential And Standard Electrode Potential standard cell potential. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Interpret electrode potentials in terms of relative. by the end of this. Standard Cell Potential And Standard Electrode Potential.
From www.youtube.com
Using Standard Electrode Potentials YouTube Standard Cell Potential And Standard Electrode Potential describe and relate the definitions of electrode and cell potentials. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any. Standard Cell Potential And Standard Electrode Potential.
From www.slideserve.com
PPT Electrochemical Cells Voltage (Electric potential) PowerPoint Standard Cell Potential And Standard Electrode Potential by the end of this section, you will be able to: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. standard cell potential. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. in electrochemistry, standard. Standard Cell Potential And Standard Electrode Potential.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Cell Potential And Standard Electrode Potential in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. standard cell potential. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. describe and relate. Standard Cell Potential And Standard Electrode Potential.
From app.pandai.org
Standard Electrode Potential Standard Cell Potential And Standard Electrode Potential describe and relate the definitions of electrode and cell potentials. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. Describe and relate the definitions of electrode and cell. Standard Cell Potential And Standard Electrode Potential.
From question.pandai.org
Standard Electrode Potential Standard Cell Potential And Standard Electrode Potential standard cell potential. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my. Standard Cell Potential And Standard Electrode Potential.
From www.slideserve.com
PPT Electrochemical Cells Voltage (Electric potential) PowerPoint Standard Cell Potential And Standard Electrode Potential Interpret electrode potentials in terms of relative. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. by the end of this. Standard Cell Potential And Standard Electrode Potential.
From cbsencertsolutiononline.blogspot.com
CBSE NCERT SOLUTIONS The standard electrode potentials at 298 K Standard Cell Potential And Standard Electrode Potential Interpret electrode potentials in terms of relative. Describe and relate the definitions of electrode and cell potentials. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. by the end of this section, you will be able to: describe and relate the definitions of electrode and cell. Standard Cell Potential And Standard Electrode Potential.
From www.tpsearchtool.com
Standard Reduction Potential Charts For Chemistry Images Standard Cell Potential And Standard Electrode Potential The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. describe and relate the definitions of electrode and cell potentials. standard cell potential. Interpret electrode potentials in terms of relative. in electrochemistry, standard electrode potential ,. Standard Cell Potential And Standard Electrode Potential.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Cell Potential And Standard Electrode Potential The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. Describe and relate the definitions of electrode and cell potentials. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Interpret electrode potentials in terms of relative. 42 rows. Standard Cell Potential And Standard Electrode Potential.
From question.pandai.org
Standard Electrode Potential Standard Cell Potential And Standard Electrode Potential Interpret electrode potentials in terms of relative. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. by the end of this section, you will be able to: revision notes on 5.3.3 standard. Standard Cell Potential And Standard Electrode Potential.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Cell Potential And Standard Electrode Potential Describe and relate the definitions of electrode and cell potentials. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The standard cell potential (\(e^o_{cell}\)) is the. Standard Cell Potential And Standard Electrode Potential.
From quizlet.com
ELECTRODE POTENTIAL AND CELL Diagram Quizlet Standard Cell Potential And Standard Electrode Potential in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. describe and relate the definitions of electrode and cell potentials. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. 42 rows calculate the standard cell potential of a voltaic cell that uses. Standard Cell Potential And Standard Electrode Potential.
From chemistryexplainedpsychokillerblogger.blogspot.com
Chemistry Explained Electrode Potential (Introduction) Standard Cell Potential And Standard Electrode Potential by the end of this section, you will be able to: Interpret electrode potentials in terms of relative. Describe and relate the definitions of electrode and cell potentials. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. describe and relate the. Standard Cell Potential And Standard Electrode Potential.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential And Standard Electrode Potential Interpret electrode potentials in terms of relative. by the end of this section, you will be able to: standard cell potential. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. describe and relate the definitions of electrode and cell potentials. in electrochemistry, standard electrode potential , or ,. Standard Cell Potential And Standard Electrode Potential.
From byjus.com
6. The standard electrode potential E^° I2/I , E^° Br /Br2 and E^° Fe Standard Cell Potential And Standard Electrode Potential standard cell potential. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. Interpret electrode potentials in terms of relative. Describe and relate the definitions of electrode and cell potentials. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. by the end. Standard Cell Potential And Standard Electrode Potential.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID4831905 Standard Cell Potential And Standard Electrode Potential describe and relate the definitions of electrode and cell potentials. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Describe and relate the definitions of electrode and cell potentials. by the end of this section, you will be able to: Interpret. Standard Cell Potential And Standard Electrode Potential.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Cell Potential And Standard Electrode Potential Interpret electrode potentials in terms of relative. by the end of this section, you will be able to: in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. Describe and relate the definitions of. Standard Cell Potential And Standard Electrode Potential.
From www.differencebetween.com
Difference Between Single Electrode Potential and Standard Electrode Standard Cell Potential And Standard Electrode Potential Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative. standard cell potential. by the end of this section, you will be able to: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. 42 rows calculate the standard cell potential of a voltaic cell that. Standard Cell Potential And Standard Electrode Potential.
From exohmihef.blob.core.windows.net
Standard Electrode Potential Meaning at Francis McQuay blog Standard Cell Potential And Standard Electrode Potential revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. by the end of this section, you will be able to: describe and relate the definitions of electrode and cell potentials. in electrochemistry, standard electrode potential , or , is a. Standard Cell Potential And Standard Electrode Potential.
From saylordotorg.github.io
Standard Potentials Standard Cell Potential And Standard Electrode Potential in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Interpret electrode potentials in terms of relative. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. . Standard Cell Potential And Standard Electrode Potential.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Standard Cell Potential And Standard Electrode Potential revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. standard cell potential. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials. Standard Cell Potential And Standard Electrode Potential.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Standard Cell Potential And Standard Electrode Potential standard cell potential. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Describe and relate the definitions of electrode and cell potentials. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. describe and. Standard Cell Potential And Standard Electrode Potential.