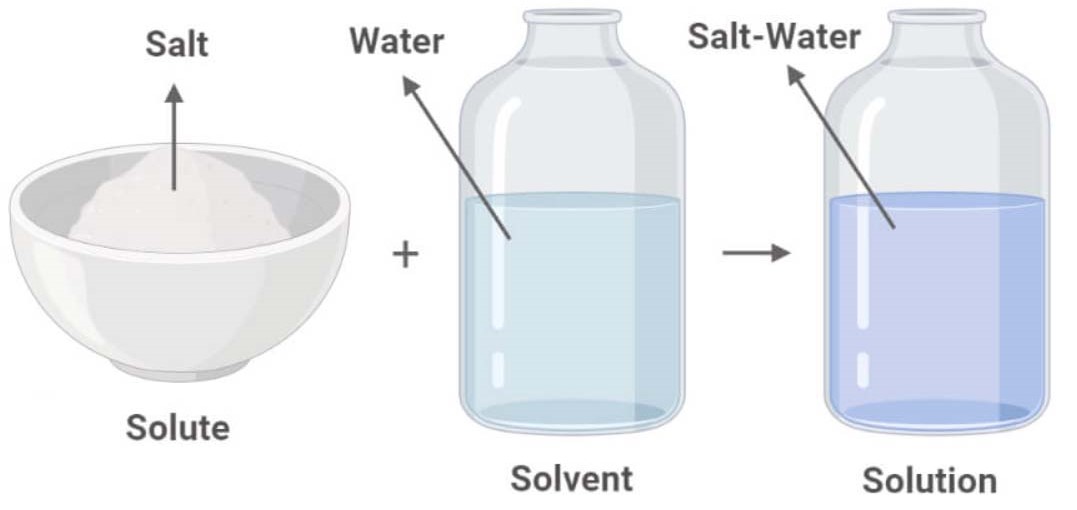
Table Salt Water What Is The Solute . In most cases, only a certain maximum amount of solute can be dissolved in. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. Water molecules move about continuously due to their. There will always be less solute than solvent. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. A solute is a molecule or particle that is distributed in a solvent. On the other hand, water vapor is considered a solute in air because nitrogen and oxygen. That means in a solution the solute is always the minor component. Salt is the solute that dissolves in water, the solvent, to form a saline solution. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a.
from educatorpages.com
That means in a solution the solute is always the minor component. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. There will always be less solute than solvent. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. Water molecules move about continuously due to their. Salt is the solute that dissolves in water, the solvent, to form a saline solution. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. On the other hand, water vapor is considered a solute in air because nitrogen and oxygen. In most cases, only a certain maximum amount of solute can be dissolved in. A solute is a molecule or particle that is distributed in a solvent.
Chemistry
Table Salt Water What Is The Solute In most cases, only a certain maximum amount of solute can be dissolved in. A solute is a molecule or particle that is distributed in a solvent. There will always be less solute than solvent. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. In most cases, only a certain maximum amount of solute can be dissolved in. Water molecules move about continuously due to their. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. That means in a solution the solute is always the minor component. On the other hand, water vapor is considered a solute in air because nitrogen and oxygen. Salt is the solute that dissolves in water, the solvent, to form a saline solution.
From www.slideserve.com
PPT BASIC’S OF SOLUTION CHEMISTRY PowerPoint Presentation, free Table Salt Water What Is The Solute Salt is the solute that dissolves in water, the solvent, to form a saline solution. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. On the other hand, water vapor is considered a solute in air because nitrogen and oxygen. Water molecules move about continuously due to their.. Table Salt Water What Is The Solute.
From saltsanity.com
How Much Sodium is in Table Salt? Tips for Low Sodium Living Table Salt Water What Is The Solute Water molecules move about continuously due to their. Salt is the solute that dissolves in water, the solvent, to form a saline solution. On the other hand, water vapor is considered a solute in air because nitrogen and oxygen. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest. Table Salt Water What Is The Solute.
From pediaa.com
What is the Difference Between Freshwater and Saltwater Table Salt Water What Is The Solute Water molecules move about continuously due to their. A solute is a molecule or particle that is distributed in a solvent. On the other hand, water vapor is considered a solute in air because nitrogen and oxygen. There will always be less solute than solvent. We will first examine the process that occurs when an ionic compound, such as table. Table Salt Water What Is The Solute.
From handletheheat.com
Kosher Salt vs. Sea Salt vs. Table Salt Handle the Heat Table Salt Water What Is The Solute Salt is the solute that dissolves in water, the solvent, to form a saline solution. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. In most cases, only a certain maximum amount of solute can be dissolved in. There. Table Salt Water What Is The Solute.
From www.slideserve.com
PPT Solute and Solvent PowerPoint Presentation, free download ID Table Salt Water What Is The Solute Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a.. Table Salt Water What Is The Solute.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? in Table Salt Water What Is The Solute Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. In most cases, only a certain maximum amount of solute can be dissolved in. Water molecules move about continuously due to their. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. That means in a solution the solute is. Table Salt Water What Is The Solute.
From shaunmwilliams.com
Chapter 11 Presentation Table Salt Water What Is The Solute There will always be less solute than solvent. That means in a solution the solute is always the minor component. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. Water molecules move about continuously due to their. On the. Table Salt Water What Is The Solute.
From educatorpages.com
Chemistry Table Salt Water What Is The Solute We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. That means in a solution the solute is always the minor component. A solute is a molecule or particle that is distributed in a solvent. There will always be less solute than solvent. Water, h 2 o, is the. Table Salt Water What Is The Solute.
From www.pinterest.com
🍃🍃Sea salt vs table salt What is better?🍃🍃 Baelwellness Salt Health Table Salt Water What Is The Solute We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. Water molecules move about continuously due to their. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount,. Table Salt Water What Is The Solute.
From jacksofscience.com
Is Salt Water a Heterogeneous Mixture? Jacks Of Science Table Salt Water What Is The Solute That means in a solution the solute is always the minor component. A solute is a molecule or particle that is distributed in a solvent. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. On the other hand, water. Table Salt Water What Is The Solute.
From foodinsight.org
What You Should Know about Sodium and Salt Food Insight Table Salt Water What Is The Solute Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. Salt is the solute that dissolves in water, the solvent, to form a saline solution. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. A solute is a molecule or. Table Salt Water What Is The Solute.
From www.todayifoundout.com
The Difference Between Kosher Salt and Table Salt Table Salt Water What Is The Solute That means in a solution the solute is always the minor component. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. Water molecules move about continuously due to their. A. Table Salt Water What Is The Solute.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Table Salt Water What Is The Solute We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. There will always be less solute than solvent. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. In. Table Salt Water What Is The Solute.
From preparatorychemistry.com
Supersaturation Table Salt Water What Is The Solute Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. There will always be less solute than solvent. On the other hand, water vapor is considered a solute in air because nitrogen and oxygen. That means in a solution the solute is always the minor component. Water molecules move about continuously due to their. Salt is the solute that dissolves in. Table Salt Water What Is The Solute.
From classnotes.org.in
Saturated Solution Class 6, Separation of Substances Table Salt Water What Is The Solute Water molecules move about continuously due to their. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. In. Table Salt Water What Is The Solute.
From handletheheat.com
Kosher Salt vs. Sea Salt vs. Table Salt Handle the Heat Table Salt Water What Is The Solute Water molecules move about continuously due to their. A solute is a molecule or particle that is distributed in a solvent. Salt is the solute that dissolves in water, the solvent, to form a saline solution. On the other hand, water vapor is considered a solute in air because nitrogen and oxygen. We will first examine the process that occurs. Table Salt Water What Is The Solute.
From www.youtube.com
How to Write the Formula for Table Salt YouTube Table Salt Water What Is The Solute That means in a solution the solute is always the minor component. Water molecules move about continuously due to their. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. There will always be less solute than solvent. On the. Table Salt Water What Is The Solute.
From www.sliderbase.com
Solutions and solubility Presentation Chemistry Table Salt Water What Is The Solute A solute is a molecule or particle that is distributed in a solvent. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. There will always be less solute than solvent. That means in a solution the solute is always. Table Salt Water What Is The Solute.
From www.thoughtco.com
Chemical Composition of Table Salt Table Salt Water What Is The Solute Water molecules move about continuously due to their. On the other hand, water vapor is considered a solute in air because nitrogen and oxygen. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. A solute is a molecule or particle that is distributed in a solvent. There will always be less solute than solvent. We will first examine the process. Table Salt Water What Is The Solute.
From sciencenotes.org
What Is a Salt in Chemistry? Definition and Examples Table Salt Water What Is The Solute In most cases, only a certain maximum amount of solute can be dissolved in. Salt is the solute that dissolves in water, the solvent, to form a saline solution. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. A solute is a molecule or particle that is distributed. Table Salt Water What Is The Solute.
From awesomehome.co
Table Salt Chemical Formula Equation Awesome Home Table Salt Water What Is The Solute That means in a solution the solute is always the minor component. Salt is the solute that dissolves in water, the solvent, to form a saline solution. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Water molecules move about continuously due to their. Water, h 2 o,. Table Salt Water What Is The Solute.
From www.pinterest.com
Curious about the differences between sea salt and table salt? Table Table Salt Water What Is The Solute That means in a solution the solute is always the minor component. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride),. Table Salt Water What Is The Solute.
From sciencing.com
What Happens When Salt Is Added to Water? Sciencing Table Salt Water What Is The Solute Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. A solute is a molecule or particle that is distributed in a solvent. Water molecules move about continuously due to their. We will first examine the process that occurs when. Table Salt Water What Is The Solute.
From www.alamy.com
Salt Water vs. Fresh Water, Infographic Stock Photo Alamy Table Salt Water What Is The Solute Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. Water molecules move about continuously due to their. There will always be less solute than solvent. That means in a solution the solute is always the minor component. We will. Table Salt Water What Is The Solute.
From ar.inspiredpencil.com
Salt And Water Solution Table Salt Water What Is The Solute We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. There will always be less solute than solvent. A solute is a molecule or particle that is distributed in a solvent. Salt is the solute that dissolves in water, the solvent, to form a saline solution. On the other. Table Salt Water What Is The Solute.
From www.thoughtco.com
Chemical Composition of Table Salt Table Salt Water What Is The Solute A solute is a molecule or particle that is distributed in a solvent. Salt is the solute that dissolves in water, the solvent, to form a saline solution. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Water, h. Table Salt Water What Is The Solute.
From www.turfcaresupply.com
JOE KNOWS! Cation Exchange Capacity Table Salt Water What Is The Solute That means in a solution the solute is always the minor component. On the other hand, water vapor is considered a solute in air because nitrogen and oxygen. There will always be less solute than solvent. A solute is a molecule or particle that is distributed in a solvent. Salt is the solute that dissolves in water, the solvent, to. Table Salt Water What Is The Solute.
From www.youtube.com
physiological salt solution types, composition and uses YouTube Table Salt Water What Is The Solute A solute is a molecule or particle that is distributed in a solvent. That means in a solution the solute is always the minor component. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. In most cases, only a certain maximum amount of solute can be dissolved in.. Table Salt Water What Is The Solute.
From techiescientist.com
Why Does Salt Make Ice Colder? Techiescientist Table Salt Water What Is The Solute Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. That means in a solution the solute is always the minor component. In most cases, only a certain maximum amount of solute can be dissolved in. Salt is the solute. Table Salt Water What Is The Solute.
From www.alamy.com
Salt water glass experiment Stock Vector Images Alamy Table Salt Water What Is The Solute Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. On the other hand, water vapor is considered a solute in air because nitrogen and oxygen. Salt is the solute that. Table Salt Water What Is The Solute.
From www.wikidoc.org
Solution wikidoc Table Salt Water What Is The Solute In most cases, only a certain maximum amount of solute can be dissolved in. A solute is a molecule or particle that is distributed in a solvent. That means in a solution the solute is always the minor component. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. There will always be less solute than solvent. On the other hand,. Table Salt Water What Is The Solute.
From philnews.ph
Salt Water Benefits What You Can Get From Drinking Warm Salt Water Table Salt Water What Is The Solute A solute is a molecule or particle that is distributed in a solvent. Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Water, h 2 o, is the solvent in this solution, as it is the chemical that is. Table Salt Water What Is The Solute.
From ar.inspiredpencil.com
Kosher Salt Crystals Table Salt Water What Is The Solute Table salt \(\left( \ce{nacl} \right)\) readily dissolves in water. A solute is a molecule or particle that is distributed in a solvent. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. That means in a solution the solute is. Table Salt Water What Is The Solute.
From slideplayer.com
Ms. Smedley & Mr. Bartolone’s ppt download Table Salt Water What Is The Solute Salt is the solute that dissolves in water, the solvent, to form a saline solution. A solute is a molecule or particle that is distributed in a solvent. Water, h 2 o, is the solvent in this solution, as it is the chemical that is present in the greatest amount, 147.2 g, and sodium chloride, nacl, is a. On the. Table Salt Water What Is The Solute.
From www.bouldersaltcompany.com
What Is the Best Salt For POTS? — Boulder Salt Company Table Salt Water What Is The Solute In most cases, only a certain maximum amount of solute can be dissolved in. There will always be less solute than solvent. That means in a solution the solute is always the minor component. On the other hand, water vapor is considered a solute in air because nitrogen and oxygen. Salt is the solute that dissolves in water, the solvent,. Table Salt Water What Is The Solute.