Is Zinc Negatively Charged . The key to writing proper ionic formulas is simple: Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. When these atoms gain electrons, they. However, as the electronegativity decreases among the halogen group, the reactivity with zinc decreases. When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell, and the zinc is attacked (oxidized to the zn 2 + ion) preferentially because of its higher electrode potential. A traditional dry cell has an outer zinc casing acting as the anode. Because the charges on the. This table shows the most common charges for atoms of the chemical elements. Zinc's electrical capabilities also extend to the most popular batteries. The total positive charge must balance the total negative charge. You can use this table to. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed.
from www.semanticscholar.org
Zinc's electrical capabilities also extend to the most popular batteries. However, as the electronegativity decreases among the halogen group, the reactivity with zinc decreases. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. A traditional dry cell has an outer zinc casing acting as the anode. You can use this table to. The total positive charge must balance the total negative charge. Because the charges on the. This table shows the most common charges for atoms of the chemical elements. The key to writing proper ionic formulas is simple: When these atoms gain electrons, they.
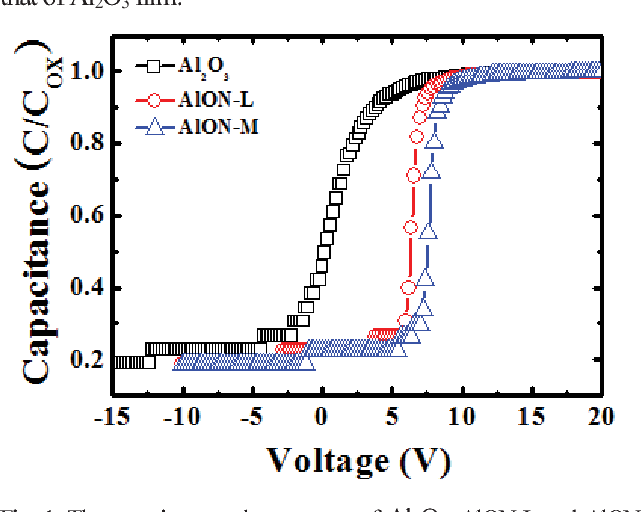
Figure 1 from High FieldEffect Mobility Amorphous IndiumTinZinc
Is Zinc Negatively Charged When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell, and the zinc is attacked (oxidized to the zn 2 + ion) preferentially because of its higher electrode potential. When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell, and the zinc is attacked (oxidized to the zn 2 + ion) preferentially because of its higher electrode potential. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Zinc's electrical capabilities also extend to the most popular batteries. This table shows the most common charges for atoms of the chemical elements. The total positive charge must balance the total negative charge. You can use this table to. A traditional dry cell has an outer zinc casing acting as the anode. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. When these atoms gain electrons, they. The key to writing proper ionic formulas is simple: Because the charges on the. However, as the electronegativity decreases among the halogen group, the reactivity with zinc decreases.
From slidetodoc.com
Electric Current Unit 3 Batteries A battery is Is Zinc Negatively Charged This table shows the most common charges for atoms of the chemical elements. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Zinc's electrical capabilities also extend to the most popular batteries. The total positive charge must balance the total negative charge. 93 rows when atoms gain electron/s,. Is Zinc Negatively Charged.
From www.researchgate.net
Energy dispersive spectrum of zinc electrode before (a) and after (b Is Zinc Negatively Charged Because the charges on the. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The total positive charge must balance the total negative charge.. Is Zinc Negatively Charged.
From www.youtube.com
How to remember the Negatively Charged Amino Acids? (aka Acidic Amino Is Zinc Negatively Charged The key to writing proper ionic formulas is simple: 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Zinc's electrical capabilities also extend to. Is Zinc Negatively Charged.
From store.drjockers.com
Zinc Charge Dr. Jockers Store Is Zinc Negatively Charged When these atoms gain electrons, they. When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell, and the zinc is attacked (oxidized to the zn 2 + ion) preferentially because of its higher electrode potential. Zinc's electrical capabilities also extend to the most popular batteries. This table shows the most common charges for atoms. Is Zinc Negatively Charged.
From www.semanticscholar.org
Figure 1 from High FieldEffect Mobility Amorphous IndiumTinZinc Is Zinc Negatively Charged A traditional dry cell has an outer zinc casing acting as the anode. When these atoms gain electrons, they. This table shows the most common charges for atoms of the chemical elements. The total positive charge must balance the total negative charge. When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell, and the. Is Zinc Negatively Charged.
From www.youtube.com
Determination of Standard Electrode potential of Zinc and Copper YouTube Is Zinc Negatively Charged Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. You can use this table to. When these atoms gain electrons, they. The key to writing proper ionic formulas is simple: The total positive charge must balance the total negative charge. A traditional dry cell has an outer zinc. Is Zinc Negatively Charged.
From www.alamy.com
Zinc atomic structure Stock Vector Images Alamy Is Zinc Negatively Charged When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell, and the zinc is attacked (oxidized to the zn 2 + ion) preferentially because of its higher electrode potential. A traditional dry cell has an outer zinc casing acting as the anode. You can use this table to. This table shows the most common. Is Zinc Negatively Charged.
From lessonschoolframboises.z14.web.core.windows.net
Reaction Of Zinc With Oxygen Is Zinc Negatively Charged A traditional dry cell has an outer zinc casing acting as the anode. When these atoms gain electrons, they. When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell, and the zinc is attacked (oxidized to the zn 2 + ion) preferentially because of its higher electrode potential. The total positive charge must balance. Is Zinc Negatively Charged.
From www.researchgate.net
Mechanisms of zinc uptake and translocation in chickpea. Plant uptake Is Zinc Negatively Charged Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The key to writing proper ionic formulas is simple: When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell, and the zinc is attacked (oxidized to the zn 2 + ion) preferentially because. Is Zinc Negatively Charged.
From www.numerade.com
SOLVED Metal binding amino acid side chains, like those the zinc Is Zinc Negatively Charged The total positive charge must balance the total negative charge. The key to writing proper ionic formulas is simple: When these atoms gain electrons, they. A traditional dry cell has an outer zinc casing acting as the anode. Because the charges on the. When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell, and. Is Zinc Negatively Charged.
From www.researchgate.net
Schematic of dendritefree alkaline zinciron flow battery. The Is Zinc Negatively Charged This table shows the most common charges for atoms of the chemical elements. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. However, as the electronegativity decreases among the halogen group, the reactivity with zinc decreases. Because the charges on the. Some atoms have nearly. Is Zinc Negatively Charged.
From valenceelectrons.com
How to Write the Electron Configuration for Zinc (Zn)? Is Zinc Negatively Charged Zinc's electrical capabilities also extend to the most popular batteries. When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell, and the zinc is attacked (oxidized to the zn 2 + ion) preferentially because of its higher electrode potential. This table shows the most common charges for atoms of the chemical elements. You can. Is Zinc Negatively Charged.
From www.scribd.com
Zinc Negatively Charge Zinc Download Free PDF Photoelectric Is Zinc Negatively Charged The key to writing proper ionic formulas is simple: You can use this table to. Zinc's electrical capabilities also extend to the most popular batteries. A traditional dry cell has an outer zinc casing acting as the anode. This table shows the most common charges for atoms of the chemical elements. However, as the electronegativity decreases among the halogen group,. Is Zinc Negatively Charged.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Zinc Have? Is Zinc Negatively Charged Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The total positive charge must balance the total negative charge. A traditional dry cell has an outer zinc casing acting as the anode. When these atoms gain electrons, they. This table shows the most common charges for atoms of. Is Zinc Negatively Charged.
From www.researchgate.net
The timeofflight mass spectrum of the negatively charged zinc oxide Is Zinc Negatively Charged However, as the electronegativity decreases among the halogen group, the reactivity with zinc decreases. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. This table shows the most common charges for atoms of the chemical elements. The key to writing proper ionic formulas is simple: When iron and. Is Zinc Negatively Charged.
From eureka.patsnap.com
Preparation method and application of metal zinc negative electrode of Is Zinc Negatively Charged This table shows the most common charges for atoms of the chemical elements. The key to writing proper ionic formulas is simple: Because the charges on the. However, as the electronegativity decreases among the halogen group, the reactivity with zinc decreases. The total positive charge must balance the total negative charge. Some atoms have nearly eight electrons in their valence. Is Zinc Negatively Charged.
From www.researchgate.net
Mechanism of coating growth on AZ91 alloy (a) negatively charged zinc Is Zinc Negatively Charged When these atoms gain electrons, they. You can use this table to. This table shows the most common charges for atoms of the chemical elements. A traditional dry cell has an outer zinc casing acting as the anode. Because the charges on the. The total positive charge must balance the total negative charge. However, as the electronegativity decreases among the. Is Zinc Negatively Charged.
From www.researchgate.net
(PDF) A 90day study of subchronic oral toxicity of 20 nm, negatively Is Zinc Negatively Charged When these atoms gain electrons, they. When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell, and the zinc is attacked (oxidized to the zn 2 + ion) preferentially because of its higher electrode potential. The key to writing proper ionic formulas is simple: Because the charges on the. The total positive charge must. Is Zinc Negatively Charged.
From slideplayer.com
Batteries How Batteries Work. ppt download Is Zinc Negatively Charged This table shows the most common charges for atoms of the chemical elements. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. However, as the electronegativity decreases among the halogen group, the reactivity with zinc decreases. You can use this table to. The key to writing proper ionic. Is Zinc Negatively Charged.
From eureka.patsnap.com
Zinc negative electrode material of zinc air cell and preparation Is Zinc Negatively Charged When these atoms gain electrons, they. This table shows the most common charges for atoms of the chemical elements. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Because the charges on the. The key to writing proper ionic formulas is simple: The total positive charge must balance. Is Zinc Negatively Charged.
From exowsidwq.blob.core.windows.net
Zinc How To Find Charge at Patel blog Is Zinc Negatively Charged The total positive charge must balance the total negative charge. Zinc's electrical capabilities also extend to the most popular batteries. When these atoms gain electrons, they. When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell, and the zinc is attacked (oxidized to the zn 2 + ion) preferentially because of its higher electrode. Is Zinc Negatively Charged.
From sciencetrends.com
What Is The Ionic Charge Of Zinc (Zn)? Science Trends Is Zinc Negatively Charged 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. This table shows the most common charges for atoms of the chemical elements. Because the charges on the. The key to writing proper ionic formulas is simple: Some atoms have nearly eight electrons in their valence. Is Zinc Negatively Charged.
From www.researchgate.net
(PDF) Negatively charged nanoporous membrane for a dendritefree Is Zinc Negatively Charged You can use this table to. However, as the electronegativity decreases among the halogen group, the reactivity with zinc decreases. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The key to writing proper ionic formulas is simple: A traditional dry cell has an outer zinc casing acting. Is Zinc Negatively Charged.
From material-properties.org
Zinc Periodic Table and Atomic Properties Is Zinc Negatively Charged The total positive charge must balance the total negative charge. This table shows the most common charges for atoms of the chemical elements. You can use this table to. However, as the electronegativity decreases among the halogen group, the reactivity with zinc decreases. A traditional dry cell has an outer zinc casing acting as the anode. Some atoms have nearly. Is Zinc Negatively Charged.
From elchoroukhost.net
Zinc Periodic Table Protons Elcho Table Is Zinc Negatively Charged This table shows the most common charges for atoms of the chemical elements. Zinc's electrical capabilities also extend to the most popular batteries. The total positive charge must balance the total negative charge. Because the charges on the. When these atoms gain electrons, they. When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell,. Is Zinc Negatively Charged.
From utedzz.blogspot.com
Periodic Table Zinc Charge Periodic Table Timeline Is Zinc Negatively Charged However, as the electronegativity decreases among the halogen group, the reactivity with zinc decreases. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. The total positive charge must balance the total negative charge. When iron and zinc together are exposed to a corrosive medium, they. Is Zinc Negatively Charged.
From www.researchgate.net
Morphologies of zinc metal (dendrite) and membrane surface. a Optical Is Zinc Negatively Charged You can use this table to. This table shows the most common charges for atoms of the chemical elements. Because the charges on the. Zinc's electrical capabilities also extend to the most popular batteries. The total positive charge must balance the total negative charge. When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell,. Is Zinc Negatively Charged.
From www.researchgate.net
Mechanism of coating growth on AZ91 alloy (a) negatively charged zinc Is Zinc Negatively Charged You can use this table to. When these atoms gain electrons, they. When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell, and the zinc is attacked (oxidized to the zn 2 + ion) preferentially because of its higher electrode potential. This table shows the most common charges for atoms of the chemical elements.. Is Zinc Negatively Charged.
From www.dreamstime.com
Zinc stock illustration. Illustration of render, chemistry 139650988 Is Zinc Negatively Charged You can use this table to. The total positive charge must balance the total negative charge. Zinc's electrical capabilities also extend to the most popular batteries. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The key to writing proper ionic formulas is simple: However, as the electronegativity. Is Zinc Negatively Charged.
From byjus.com
The diagram shows a beaker containing a solution of zinc sulphate and Is Zinc Negatively Charged You can use this table to. When these atoms gain electrons, they. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. The total positive charge must balance the total negative charge. When iron and zinc together are exposed to a corrosive medium, they constitute an. Is Zinc Negatively Charged.
From www.researchgate.net
A Long‐Life Manganese Oxide Cathode Material for Aqueous Zinc Batteries Is Zinc Negatively Charged The total positive charge must balance the total negative charge. This table shows the most common charges for atoms of the chemical elements. Because the charges on the. When these atoms gain electrons, they. Zinc's electrical capabilities also extend to the most popular batteries. A traditional dry cell has an outer zinc casing acting as the anode. You can use. Is Zinc Negatively Charged.
From www.alamy.com
Early battery, working by chemical reaction, with zinc strip as Is Zinc Negatively Charged A traditional dry cell has an outer zinc casing acting as the anode. The key to writing proper ionic formulas is simple: 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. You can use this table to. Because the charges on the. Some atoms have. Is Zinc Negatively Charged.
From zinc.ca
Zinc and iron deficiency Zinc.ca Is Zinc Negatively Charged However, as the electronegativity decreases among the halogen group, the reactivity with zinc decreases. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The total positive charge must balance the total negative charge. A traditional dry cell has an outer zinc casing acting as the anode. When iron. Is Zinc Negatively Charged.
From slideplayer.com
fundamental forces + sweet physics ppt download Is Zinc Negatively Charged Because the charges on the. A traditional dry cell has an outer zinc casing acting as the anode. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. When iron and zinc together are exposed to a corrosive medium, they constitute an electrolytic cell, and the zinc is attacked. Is Zinc Negatively Charged.
From www.youtube.com
Ionic Charge for Zinc (Zn) YouTube Is Zinc Negatively Charged This table shows the most common charges for atoms of the chemical elements. 93 rows when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. You can use this table to. Because the charges on the. Zinc's electrical capabilities also extend to the most popular batteries. Some atoms. Is Zinc Negatively Charged.