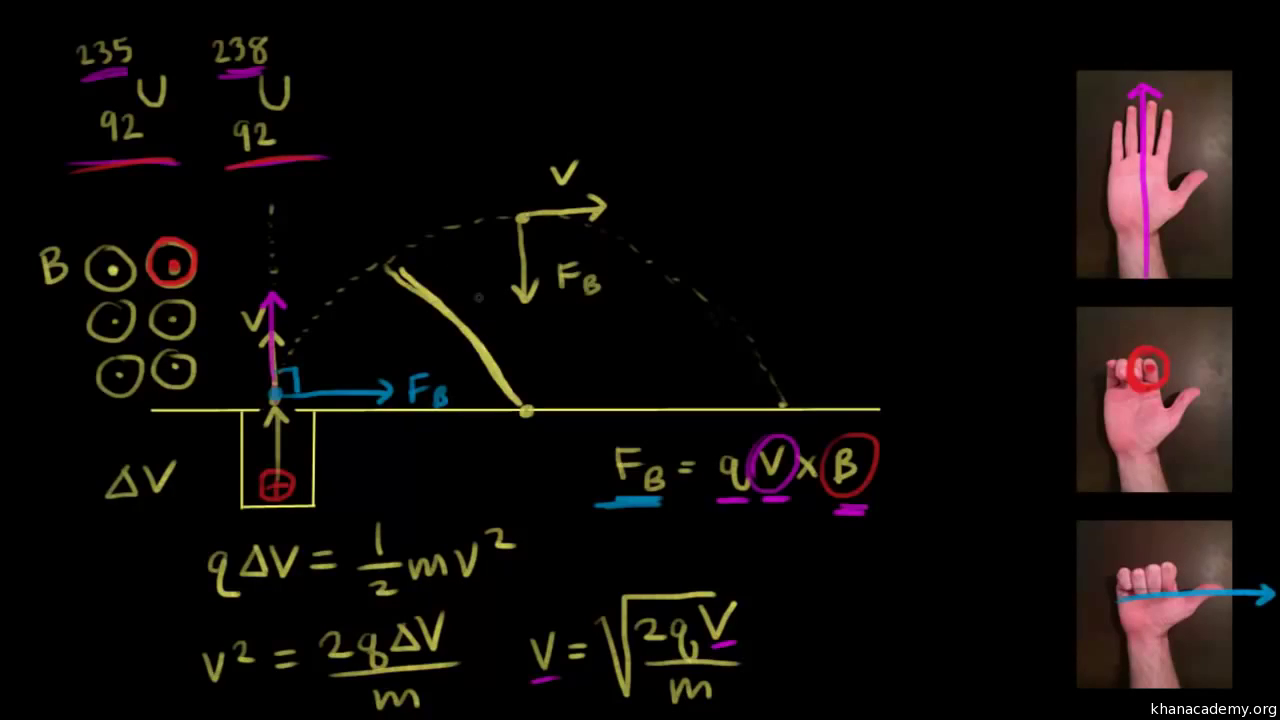
Spectrometer Equation . Time of flight mass spectrometry. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. It is the most useful instrument for accurate determination of the. Let “y” equal the concentration. Use the equation (y=mx+b) to determine concentration of samples. Mass spectrometry is a powerful analytical technique.
from www.tessshebaylo.com
We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Let “y” equal the concentration. Time of flight mass spectrometry. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) Mass spectrometry is a powerful analytical technique. Use the equation (y=mx+b) to determine concentration of samples. It is the most useful instrument for accurate determination of the.
Mass Spectrometer Equation Derivation Tessshebaylo
Spectrometer Equation We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Mass spectrometry is a powerful analytical technique. Use the equation (y=mx+b) to determine concentration of samples. It is the most useful instrument for accurate determination of the. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Let “y” equal the concentration. Time of flight mass spectrometry. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\)
From www.science-revision.co.uk
TOF mass spectrometer Spectrometer Equation Let “y” equal the concentration. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could. Spectrometer Equation.
From www.youtube.com
Mass spectrometryMass spectroscopyMass spectrometerPrinciple Spectrometer Equation Use the equation (y=mx+b) to determine concentration of samples. Time of flight mass spectrometry. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions,. Spectrometer Equation.
From www.slideshare.net
Atomic Spectroscopy Basic Principles and Instruments Spectrometer Equation Mass spectrometry is a powerful analytical technique. Use the equation (y=mx+b) to determine concentration of samples. It is the most useful instrument for accurate determination of the. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2}. Spectrometer Equation.
From www.numerade.com
Calculation of the Coupling Constant; This is a simple example that Spectrometer Equation Let “y” equal the concentration. It is the most useful instrument for accurate determination of the. Use the equation (y=mx+b) to determine concentration of samples. Mass spectrometry is a powerful analytical technique. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons.. Spectrometer Equation.
From www.chemistrystudent.com
Mass Spectrometry (ALevel) ChemistryStudent Spectrometer Equation Let “y” equal the concentration. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Use the equation (y=mx+b) to determine concentration of samples. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions,. Spectrometer Equation.
From www.tessshebaylo.com
Mass Spectrometer Equation Voltage Tessshebaylo Spectrometer Equation Time of flight mass spectrometry. Use the equation (y=mx+b) to determine concentration of samples. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions,. Spectrometer Equation.
From crunchchemistry.co.uk
Time of flight mass spectrometer Crunch Chemistry Spectrometer Equation In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen. Spectrometer Equation.
From www.tessshebaylo.com
Mass Spectrometer Equation Voltage Tessshebaylo Spectrometer Equation We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. It is the most useful instrument for accurate determination of the. Use the equation (y=mx+b) to determine concentration of samples. Let “y” equal the concentration. Mass spectrometry is a powerful analytical technique.. Spectrometer Equation.
From znanio.ru
14Motion of a charged particle in the field Spectrometer Equation We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Use the equation (y=mx+b) to determine concentration of samples. Time of flight mass spectrometry. Let “y” equal the concentration. In this equation, b is the magnetic field strength, v is the kinetic. Spectrometer Equation.
From www.tessshebaylo.com
Mass Spectrometer Equation Tessshebaylo Spectrometer Equation Let “y” equal the concentration. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Use the equation (y=mx+b) to determine concentration of samples. Time of flight mass spectrometry. In this equation, b is the magnetic field strength, v is the kinetic. Spectrometer Equation.
From www.pinterest.com
Khan Academy Mass spectrometry, Spectrometers, Physics and mathematics Spectrometer Equation Time of flight mass spectrometry. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) Use the equation (y=mx+b) to determine concentration of samples. It is the most useful instrument for accurate determination of. Spectrometer Equation.
From www.tessshebaylo.com
Mass Spectrometer Equation Voltage Tessshebaylo Spectrometer Equation Use the equation (y=mx+b) to determine concentration of samples. Mass spectrometry is a powerful analytical technique. Time of flight mass spectrometry. Let “y” equal the concentration. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2}. Spectrometer Equation.
From datespeck.com
Mass Spectrometry Fundamentals & Principles Datespeck Spectrometer Equation In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen. Spectrometer Equation.
From ibsen.com
Spectrometer design guide Ibsen Photonics Spectrometer Equation Time of flight mass spectrometry. It is the most useful instrument for accurate determination of the. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Let “y” equal the concentration. Mass spectrometry is a powerful analytical technique. Use the equation (y=mx+b). Spectrometer Equation.
From www.youtube.com
Mass Spectrometer Calculations, Paper 1 AQA A Level Chemistry YouTube Spectrometer Equation In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) Let “y” equal the concentration. Mass spectrometry is a powerful analytical technique. We developed the bohr model for the hydrogen atom (section 6.3.3) that. Spectrometer Equation.
From wavelength-oe.com
What is a Spectrometer? UV, VIS and IR Spectrometer Explained Spectrometer Equation Let “y” equal the concentration. Use the equation (y=mx+b) to determine concentration of samples. Mass spectrometry is a powerful analytical technique. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) Time of flight. Spectrometer Equation.
From www.science-revision.co.uk
TOF mass spectrometer Spectrometer Equation Time of flight mass spectrometry. It is the most useful instrument for accurate determination of the. Mass spectrometry is a powerful analytical technique. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. In this equation, b is the magnetic field strength,. Spectrometer Equation.
From www.numerade.com
SOLVED A mass spectrometer accelerates a charge q through a potential Spectrometer Equation In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) Time of flight mass spectrometry. Use the equation (y=mx+b) to determine concentration of samples. It is the most useful instrument for accurate determination of. Spectrometer Equation.
From www.optecks.com
Spectroscopy Introduction and Traditional Methods Spectrometer Equation In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) Time of flight mass spectrometry. It is the most useful instrument for accurate determination of the. Use the equation (y=mx+b) to determine concentration of. Spectrometer Equation.
From www.showme.com
Mass Spectrometer Science ShowMe Spectrometer Equation Time of flight mass spectrometry. Let “y” equal the concentration. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the. Spectrometer Equation.
From www.tessshebaylo.com
Mass Spectrometer Equation Derivation Tessshebaylo Spectrometer Equation Time of flight mass spectrometry. Use the equation (y=mx+b) to determine concentration of samples. Mass spectrometry is a powerful analytical technique. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) Let “y” equal. Spectrometer Equation.
From www.tessshebaylo.com
Mass Spectrometer Equation Derivation Tessshebaylo Spectrometer Equation Mass spectrometry is a powerful analytical technique. Let “y” equal the concentration. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r. Spectrometer Equation.
From www.tessshebaylo.com
Mass Spectrometer Equation Voltage Tessshebaylo Spectrometer Equation We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Mass spectrometry is a powerful analytical technique. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature. Spectrometer Equation.
From www.youtube.com
Mass Spectrometer Problem YouTube Spectrometer Equation Use the equation (y=mx+b) to determine concentration of samples. Mass spectrometry is a powerful analytical technique. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) Time of flight mass spectrometry. Let “y” equal. Spectrometer Equation.
From www.tessshebaylo.com
Mass Spectrometer Equation Derivation Tessshebaylo Spectrometer Equation Let “y” equal the concentration. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the. Spectrometer Equation.
From www.tessshebaylo.com
Quadrupole Mass Spectrometer Equation Tessshebaylo Spectrometer Equation Use the equation (y=mx+b) to determine concentration of samples. Let “y” equal the concentration. It is the most useful instrument for accurate determination of the. Mass spectrometry is a powerful analytical technique. Time of flight mass spectrometry. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of. Spectrometer Equation.
From www.tessshebaylo.com
Mass Spectrometry Equation Chemistry Tessshebaylo Spectrometer Equation Mass spectrometry is a powerful analytical technique. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature. Spectrometer Equation.
From www.linquip.com
Want To Know Everything About Spectrometer vs Spectrophotometer? Now Spectrometer Equation Time of flight mass spectrometry. It is the most useful instrument for accurate determination of the. Let “y” equal the concentration. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) Mass spectrometry is. Spectrometer Equation.
From www.tessshebaylo.com
Mass Spectrometer Equation Voltage Tessshebaylo Spectrometer Equation Time of flight mass spectrometry. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Use the equation (y=mx+b) to determine concentration of samples. It is the most useful instrument for accurate determination of the. Let “y” equal the concentration. Mass spectrometry. Spectrometer Equation.
From www.tessshebaylo.com
Mass Spectroscopy Equation Tessshebaylo Spectrometer Equation Let “y” equal the concentration. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Use the equation (y=mx+b) to determine concentration of samples. It is the most useful instrument for accurate determination of the. Mass spectrometry is a powerful analytical technique.. Spectrometer Equation.
From www.tessshebaylo.com
Mass Spectrometer Equation Tessshebaylo Spectrometer Equation It is the most useful instrument for accurate determination of the. Use the equation (y=mx+b) to determine concentration of samples. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) We developed the bohr. Spectrometer Equation.
From www.tessshebaylo.com
Mass Spectrometer Equation Voltage Tessshebaylo Spectrometer Equation We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field. Spectrometer Equation.
From support.zemax.com
How to build a spectrometer theory Knowledgebase Spectrometer Equation It is the most useful instrument for accurate determination of the. Let “y” equal the concentration. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) Use the equation (y=mx+b) to determine concentration of. Spectrometer Equation.
From chemistrymadesimple.net
How Does A Mass Spectrometer Work? Chemistry Made Simple Spectrometer Equation Mass spectrometry is a powerful analytical technique. Time of flight mass spectrometry. Let “y” equal the concentration. Use the equation (y=mx+b) to determine concentration of samples. It is the most useful instrument for accurate determination of the. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of. Spectrometer Equation.
From www.tessshebaylo.com
Mass Spectrometer Equation Tessshebaylo Spectrometer Equation Time of flight mass spectrometry. In this equation, b is the magnetic field strength, v is the kinetic energy of the entering ions, and r is the radius of curvature for the path through the magnetic field \(\frac{m}{ze}=\frac{b^{2} r^{2} }{2 v}\) Let “y” equal the concentration. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the. Spectrometer Equation.