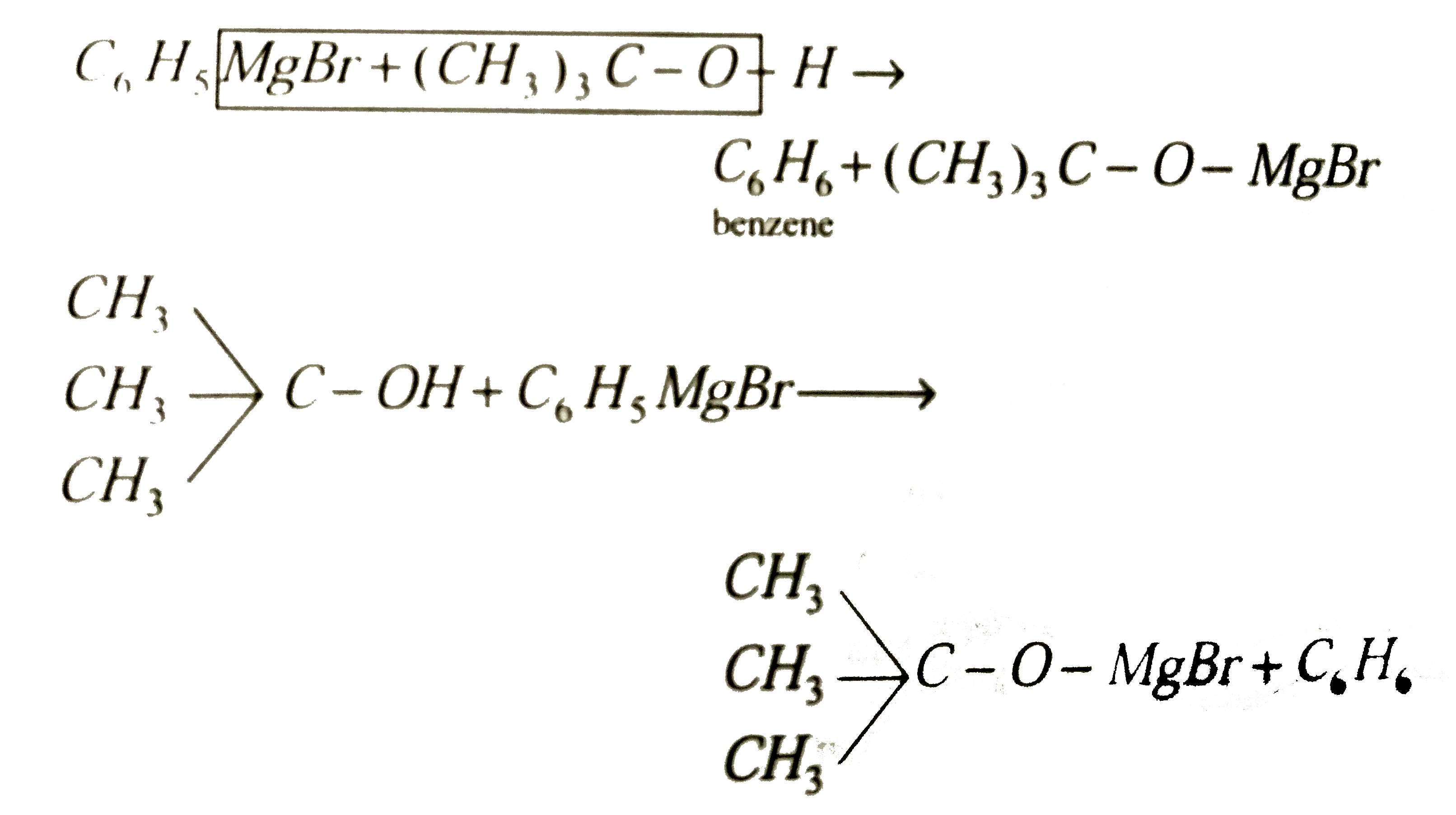When N-Butyl Magnesium Iodide Reacts With Water The Product Is . If you're behind a web filter, please. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. Explain why phenols are more acidic than alcohols. The order of reactivity of alcohols is 3° > 2° > 1° methyl. The most common wash in separatory funnels is probably water. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. (i) explain why the solution obtained is alkaline. Explain, in terms of inductive and resonance effects, why a given substituted. (c) when sodium oxide reacts with water an alkaline solution is obtained. If you're seeing this message, it means we're having trouble loading external resources on our website.
from www.doubtnut.com
(i) explain why the solution obtained is alkaline. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. Explain why phenols are more acidic than alcohols. If you're seeing this message, it means we're having trouble loading external resources on our website. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. The most common wash in separatory funnels is probably water. If you're behind a web filter, please. The order of reactivity of alcohols is 3° > 2° > 1° methyl. (c) when sodium oxide reacts with water an alkaline solution is obtained. Explain, in terms of inductive and resonance effects, why a given substituted.
When phenyl magnesium bromide reacts with tbutanol, the product would
When N-Butyl Magnesium Iodide Reacts With Water The Product Is Explain, in terms of inductive and resonance effects, why a given substituted. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. (i) explain why the solution obtained is alkaline. If you're seeing this message, it means we're having trouble loading external resources on our website. (c) when sodium oxide reacts with water an alkaline solution is obtained. Explain, in terms of inductive and resonance effects, why a given substituted. If you're behind a web filter, please. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. The order of reactivity of alcohols is 3° > 2° > 1° methyl. Explain why phenols are more acidic than alcohols. The most common wash in separatory funnels is probably water.
From www.doubtnut.com
Ethyl magnesium iodide reacts with propylamine to give When N-Butyl Magnesium Iodide Reacts With Water The Product Is If you're behind a web filter, please. The order of reactivity of alcohols is 3° > 2° > 1° methyl. Explain, in terms of inductive and resonance effects, why a given substituted. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. If you're seeing this message, it. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.toppr.com
What happens when methyl magnesium bromide is treated with formaldehyde When N-Butyl Magnesium Iodide Reacts With Water The Product Is (i) explain why the solution obtained is alkaline. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. The order of reactivity of alcohols is 3° > 2° > 1° methyl. If you're behind a web filter, please. Explain, in terms of inductive and resonance effects, why a. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.toppr.com
quuuuh? What is the need to balance a chemical equation? 7 What happens When N-Butyl Magnesium Iodide Reacts With Water The Product Is The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. (c) when sodium oxide reacts with water an alkaline solution is obtained. The most common wash in separatory funnels is probably water. The order of reactivity of alcohols is 3° > 2° > 1° methyl. If you're behind a web filter, please. Explain why. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.toppr.com
What happens when(i) isopropyl bromide is heated with alcoholic KOH;(ii When N-Butyl Magnesium Iodide Reacts With Water The Product Is Explain, in terms of inductive and resonance effects, why a given substituted. (c) when sodium oxide reacts with water an alkaline solution is obtained. The most common wash in separatory funnels is probably water. Explain why phenols are more acidic than alcohols. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.numerade.com
Magnesium carbonate into magnesium When N-Butyl Magnesium Iodide Reacts With Water The Product Is If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. Explain, in terms of inductive and resonance effects, why a given substituted. Explain why phenols. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.chegg.com
Solved When magnesium metal (Mg) reacts with hydrochloric When N-Butyl Magnesium Iodide Reacts With Water The Product Is I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. The order of reactivity of alcohols is 3° > 2° > 1° methyl. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. (c) when sodium oxide reacts with water an alkaline. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.chegg.com
Solved 10.Tertbutyl iodide reacts with methanol via SN1 When N-Butyl Magnesium Iodide Reacts With Water The Product Is Explain, in terms of inductive and resonance effects, why a given substituted. Explain why phenols are more acidic than alcohols. (c) when sodium oxide reacts with water an alkaline solution is obtained. (i) explain why the solution obtained is alkaline. The most common wash in separatory funnels is probably water. If you're seeing this message, it means we're having trouble. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.doubtnut.com
When phenyl magnesium bromide reacts with tbutanol, the product would When N-Butyl Magnesium Iodide Reacts With Water The Product Is I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. If you're seeing this message, it means we're having trouble loading external resources on our website. The most common wash in separatory funnels is probably water. Explain why phenols are more acidic than alcohols. If you're behind a. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.numerade.com
SOLVED 30 g of nbutyl bromide were obtained by brominating 25 g of n When N-Butyl Magnesium Iodide Reacts With Water The Product Is I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. (i) explain why the solution obtained is alkaline. If you're seeing this message, it means we're having trouble loading external resources on. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.chegg.com
Solved Lead (II) nitrate reacts with sodium iodide to When N-Butyl Magnesium Iodide Reacts With Water The Product Is The most common wash in separatory funnels is probably water. (i) explain why the solution obtained is alkaline. Explain, in terms of inductive and resonance effects, why a given substituted. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. The order of reactivity of the hydrogen halides. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From byjus.com
9. Which of the following pathways produces 2 hexanone? (i) 1 Hexyne is When N-Butyl Magnesium Iodide Reacts With Water The Product Is Explain, in terms of inductive and resonance effects, why a given substituted. The most common wash in separatory funnels is probably water. If you're seeing this message, it means we're having trouble loading external resources on our website. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup.. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.toppr.com
ethyl tertbutyl ketone D water o followed by 122. Which of the When N-Butyl Magnesium Iodide Reacts With Water The Product Is If you're behind a web filter, please. The order of reactivity of alcohols is 3° > 2° > 1° methyl. (c) when sodium oxide reacts with water an alkaline solution is obtained. If you're seeing this message, it means we're having trouble loading external resources on our website. Explain, in terms of inductive and resonance effects, why a given substituted.. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.toppr.com
ethyl tertbutyl ketone D water o followed by 122. Which of the When N-Butyl Magnesium Iodide Reacts With Water The Product Is If you're behind a web filter, please. The order of reactivity of alcohols is 3° > 2° > 1° methyl. Explain, in terms of inductive and resonance effects, why a given substituted. (c) when sodium oxide reacts with water an alkaline solution is obtained. The most common wash in separatory funnels is probably water. If you're seeing this message, it. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.numerade.com
SOLVED Magnesium reacts with iodine gas at high temperatures to form When N-Butyl Magnesium Iodide Reacts With Water The Product Is The most common wash in separatory funnels is probably water. (i) explain why the solution obtained is alkaline. Explain why phenols are more acidic than alcohols. (c) when sodium oxide reacts with water an alkaline solution is obtained. If you're seeing this message, it means we're having trouble loading external resources on our website. I’m being ask to show the. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.meritnation.com
why calcium and magnesium floats on water Science Metals and Non When N-Butyl Magnesium Iodide Reacts With Water The Product Is Explain, in terms of inductive and resonance effects, why a given substituted. If you're behind a web filter, please. (i) explain why the solution obtained is alkaline. The most common wash in separatory funnels is probably water. The order of reactivity of alcohols is 3° > 2° > 1° methyl. If you're seeing this message, it means we're having trouble. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.doubtnut.com
Formaldehyde is treated with methyl magnesium iodide in dry ether and When N-Butyl Magnesium Iodide Reacts With Water The Product Is (i) explain why the solution obtained is alkaline. If you're seeing this message, it means we're having trouble loading external resources on our website. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. (c) when sodium oxide reacts with water an alkaline solution is obtained. The most common wash in separatory funnels is. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.numerade.com
SOLVED Write the balanced equation for Magnesium nitride + water When N-Butyl Magnesium Iodide Reacts With Water The Product Is I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. The order of reactivity of alcohols is 3° > 2° > 1° methyl. (i) explain why the solution obtained is alkaline. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. If. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.toppr.com
4) HYU . 3) Wurtz reaction 4) Ether Methyl magnesium iodide reacts with When N-Butyl Magnesium Iodide Reacts With Water The Product Is The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. If you're behind a web filter, please. The order of reactivity of alcohols is 3° > 2° > 1° methyl. (c) when sodium oxide reacts with water an alkaline solution is obtained. Explain why phenols are more acidic than alcohols. I’m being ask to. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.numerade.com
SOLVEDAn organic compound A reacts with methyl magnesium iodide to When N-Butyl Magnesium Iodide Reacts With Water The Product Is The order of reactivity of alcohols is 3° > 2° > 1° methyl. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. Explain why phenols are more acidic than alcohols. Explain, in terms of inductive and resonance effects, why a given substituted. (c) when sodium oxide reacts with water an alkaline solution is. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.toppr.com
When phenyl magnesium bromide reacts with tertiary butyl alcohol, the When N-Butyl Magnesium Iodide Reacts With Water The Product Is (i) explain why the solution obtained is alkaline. The order of reactivity of alcohols is 3° > 2° > 1° methyl. If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please. (c) when sodium oxide reacts with water an alkaline solution is obtained. I’m being ask to. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.numerade.com
SOLVEDFrom which of the following tertiary butyl alcohol is obtained When N-Butyl Magnesium Iodide Reacts With Water The Product Is (c) when sodium oxide reacts with water an alkaline solution is obtained. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. If you're seeing this message, it means we're having trouble loading external resources on our website. Explain, in terms of inductive and resonance effects, why a given substituted. If you're behind a. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.toppr.com
Methyl magnesium iodide reacts with ethyl alcohol to produce When N-Butyl Magnesium Iodide Reacts With Water The Product Is (c) when sodium oxide reacts with water an alkaline solution is obtained. (i) explain why the solution obtained is alkaline. The order of reactivity of alcohols is 3° > 2° > 1° methyl. Explain why phenols are more acidic than alcohols. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.toppr.com
What happens when(i) n butyl chloride is treated with alcoholic KOH When N-Butyl Magnesium Iodide Reacts With Water The Product Is Explain, in terms of inductive and resonance effects, why a given substituted. The order of reactivity of alcohols is 3° > 2° > 1° methyl. If you're seeing this message, it means we're having trouble loading external resources on our website. Explain why phenols are more acidic than alcohols. (c) when sodium oxide reacts with water an alkaline solution is. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From brainly.in
Which of the following compounds gives 2methyl propan2ol by the When N-Butyl Magnesium Iodide Reacts With Water The Product Is (i) explain why the solution obtained is alkaline. If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. I’m being ask to show the products of a carboxylate salt (ethyl butanoate). When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.doubtnut.com
Ethyl acetate is obtained when methyl magnesium iodide reacts with When N-Butyl Magnesium Iodide Reacts With Water The Product Is If you're seeing this message, it means we're having trouble loading external resources on our website. (i) explain why the solution obtained is alkaline. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. The order of reactivity of the hydrogen halides is hi > hbr > hcl. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From byjus.com
Phenyl magnesium bromide reacts with methanol to give When N-Butyl Magnesium Iodide Reacts With Water The Product Is The order of reactivity of alcohols is 3° > 2° > 1° methyl. Explain why phenols are more acidic than alcohols. Explain, in terms of inductive and resonance effects, why a given substituted. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. The most common wash in. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.numerade.com
SOLVED Part F Mixed Reactions Lithium hydroxide solution is the When N-Butyl Magnesium Iodide Reacts With Water The Product Is The order of reactivity of alcohols is 3° > 2° > 1° methyl. Explain, in terms of inductive and resonance effects, why a given substituted. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. The most common wash in separatory funnels is probably water. Explain why phenols are more acidic than alcohols. (i). When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.toppr.com
ethyl tertbutyl ketone D water o followed by 122. Which of the When N-Butyl Magnesium Iodide Reacts With Water The Product Is The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. If you're behind a web filter, please. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. (c) when sodium oxide reacts with water an alkaline solution is obtained. Explain why phenols. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From byjus.com
9. Which of the following pathways produces 2 hexanone? (i) 1 Hexyne is When N-Butyl Magnesium Iodide Reacts With Water The Product Is The most common wash in separatory funnels is probably water. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. Explain, in terms of inductive and resonance effects, why a given substituted. (i) explain why the solution obtained is alkaline. If you're behind a web filter, please. (c). When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.numerade.com
SOLVED Texts 25. Magnesium sulfate reacts with lithium hydroxide. 26 When N-Butyl Magnesium Iodide Reacts With Water The Product Is The most common wash in separatory funnels is probably water. The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. Explain why phenols are more acidic than alcohols. If you're seeing this message, it means we're having trouble loading external resources on our website. (i) explain why the solution obtained is alkaline. I’m being. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.doubtnut.com
[Odia] When nbutyl magnesium iodide is treated with water the product When N-Butyl Magnesium Iodide Reacts With Water The Product Is The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. The most common wash in separatory funnels is probably water. If you're behind a web filter, please. (i) explain why the solution obtained is alkaline. Explain why phenols are more acidic than alcohols. Explain, in terms of inductive and resonance effects, why a given. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.vedantu.com
The reaction of methyl magnesium iodide with acetone followed by When N-Butyl Magnesium Iodide Reacts With Water The Product Is The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. If you're behind a web filter, please. The most common wash in separatory funnels is probably water. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. The order of reactivity of. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.toppr.com
() 1Hexyne is treated with Hg2®/dil. H,SO (ii) 3Methylhept2ene is When N-Butyl Magnesium Iodide Reacts With Water The Product Is Explain, in terms of inductive and resonance effects, why a given substituted. The order of reactivity of alcohols is 3° > 2° > 1° methyl. (c) when sodium oxide reacts with water an alkaline solution is obtained. I’m being ask to show the products of a carboxylate salt (ethyl butanoate) and a methyl grignard followed by a water workup. If. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From byjus.com
Identify the products obtained when hot hydrogen iodide react with When N-Butyl Magnesium Iodide Reacts With Water The Product Is (i) explain why the solution obtained is alkaline. (c) when sodium oxide reacts with water an alkaline solution is obtained. If you're seeing this message, it means we're having trouble loading external resources on our website. Explain, in terms of inductive and resonance effects, why a given substituted. If you're behind a web filter, please. The order of reactivity of. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.
From www.youtube.com
Reaction between Magnesium and Water (Mg + H2O) YouTube When N-Butyl Magnesium Iodide Reacts With Water The Product Is The order of reactivity of the hydrogen halides is hi > hbr > hcl (hf is. The most common wash in separatory funnels is probably water. Explain, in terms of inductive and resonance effects, why a given substituted. (c) when sodium oxide reacts with water an alkaline solution is obtained. Explain why phenols are more acidic than alcohols. If you're. When N-Butyl Magnesium Iodide Reacts With Water The Product Is.