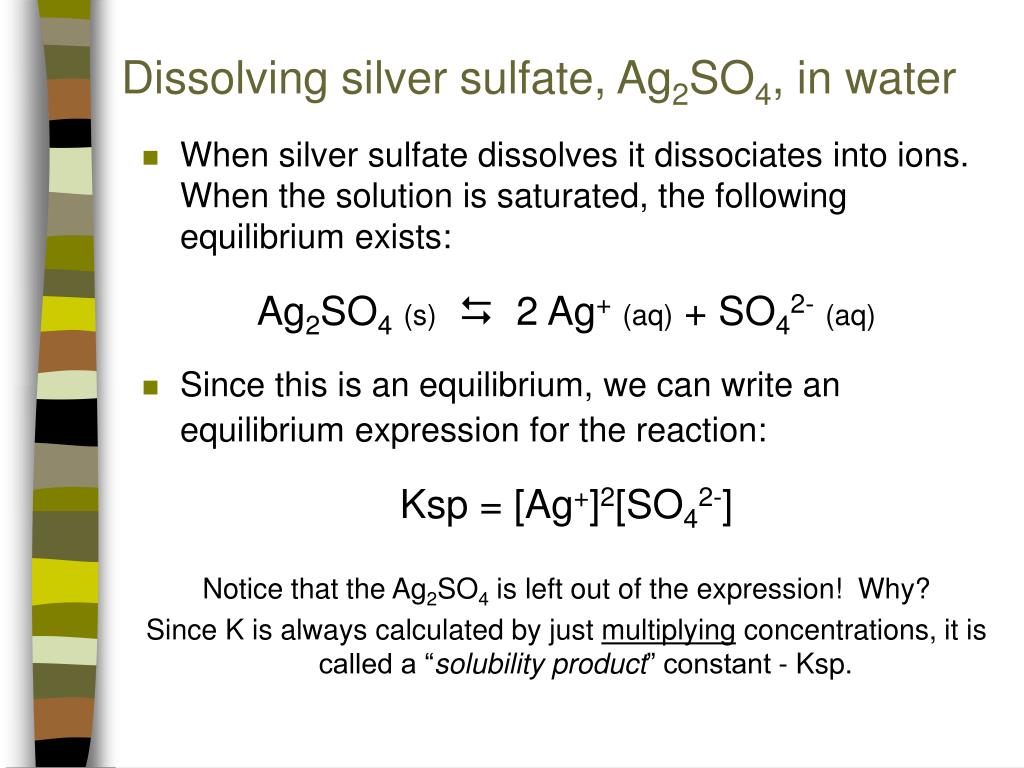
Solubility Equilibria Lab . The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. You will test the effects of changing the solution volume and temperature on the solubility. When a substance such as sodium chloride is added to water, it dissolves, forming an aqueous. The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in water. The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. There are a number of patterns in the data obtained. We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of solvent.
from www.slideserve.com
The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. You will test the effects of changing the solution volume and temperature on the solubility. We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. There are a number of patterns in the data obtained. The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of solvent. The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in water. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. When a substance such as sodium chloride is added to water, it dissolves, forming an aqueous.
PPT Solubility Equilibria PowerPoint Presentation, free download ID
Solubility Equilibria Lab There are a number of patterns in the data obtained. You will test the effects of changing the solution volume and temperature on the solubility. When a substance such as sodium chloride is added to water, it dissolves, forming an aqueous. There are a number of patterns in the data obtained. The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of solvent. We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in water.
From www.slideserve.com
PPT Chapter 18 Part 2 Solubility and Complexation Equilibria Solubility Equilibria Lab You will test the effects of changing the solution volume and temperature on the solubility. There are a number of patterns in the data obtained. We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. The amount of salt that must be added to a given volume of solvent to form a saturated. Solubility Equilibria Lab.
From www.youtube.com
Solubility Equilibria Common Ion Effect. YouTube Solubility Equilibria Lab You will test the effects of changing the solution volume and temperature on the solubility. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of. Solubility Equilibria Lab.
From slideplayer.com
17.4 Solubility Equilibria ppt download Solubility Equilibria Lab There are a number of patterns in the data obtained. The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of solvent. When a substance such as sodium chloride is added to water, it dissolves, forming an aqueous. The amount of salt that must be added to. Solubility Equilibria Lab.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID307857 Solubility Equilibria Lab The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. When a substance such as sodium chloride is added to water, it dissolves, forming an aqueous. The solubility product constant, kₛₚ, is. Solubility Equilibria Lab.
From www.scribd.com
Chapter 16 Solubility and Complex Ion Equilibria Solubility Solubility Equilibria Lab When a substance such as sodium chloride is added to water, it dissolves, forming an aqueous. The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of solvent. We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. The solubility. Solubility Equilibria Lab.
From www.slideserve.com
PPT Chapter 18 Part 2 Solubility and Complexation Equilibria Solubility Equilibria Lab There are a number of patterns in the data obtained. The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of. Solubility Equilibria Lab.
From www.studocu.com
Lab 10 Solubility Equilibria Name Partner Student No Student No Solubility Equilibria Lab You will test the effects of changing the solution volume and temperature on the solubility. We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. There are a number of patterns in the data obtained. The amount of salt that must be added to a given volume of solvent to form a saturated. Solubility Equilibria Lab.
From slidetodoc.com
AcidBase Equilibria and Solubility Equilibria Chapter 16 1 Solubility Equilibria Lab The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in water. When a substance such as sodium chloride is added to water, it dissolves, forming an aqueous. You will test the effects of changing the solution volume and temperature on the solubility. The equilibrium constant for a dissolution reaction, called. Solubility Equilibria Lab.
From www.youtube.com
Introduction to solubility equilibria Equilibrium AP Chemistry Solubility Equilibria Lab We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in water. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. The. Solubility Equilibria Lab.
From www.slideserve.com
PPT Solubility Equilibria PowerPoint Presentation, free download ID Solubility Equilibria Lab The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in water. When a substance such as sodium chloride is added to water, it dissolves, forming an aqueous. The solubility. Solubility Equilibria Lab.
From www.youtube.com
PhET salts and solubility equilibria YouTube Solubility Equilibria Lab You will test the effects of changing the solution volume and temperature on the solubility. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. When a substance such as sodium chloride. Solubility Equilibria Lab.
From www.youtube.com
Solubility Equilibria Solubility Product. YouTube Solubility Equilibria Lab The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. When a substance such as sodium chloride is added to water, it dissolves, forming an aqueous. The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume. Solubility Equilibria Lab.
From www.slideserve.com
PPT Solubility Equilibria PowerPoint Presentation, free download ID Solubility Equilibria Lab The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in water. When a substance such as sodium chloride is added to water, it dissolves,. Solubility Equilibria Lab.
From www.studocu.com
Solubility Equilibrium Lab Report Name Sami Abboud Klink Partner Solubility Equilibria Lab We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. There are a number of patterns in the data obtained. You will test the effects of changing. Solubility Equilibria Lab.
From courses.lumenlearning.com
Solubility Equilibria Boundless Chemistry Solubility Equilibria Lab The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of solvent. We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. When a substance such as sodium chloride is added to water, it dissolves, forming an aqueous. There are. Solubility Equilibria Lab.
From www.slideserve.com
PPT Solubility Equilibria PowerPoint Presentation, free download ID Solubility Equilibria Lab The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of solvent. The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in water. There are a number of patterns in the data obtained. We begin our discussion. Solubility Equilibria Lab.
From www.studypool.com
SOLUTION Chemistry acid and base equilibria and solubility equilibria Solubility Equilibria Lab When a substance such as sodium chloride is added to water, it dissolves, forming an aqueous. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. You will test the effects of changing the solution volume and temperature on the solubility. We begin our discussion of solubility and complexation. Solubility Equilibria Lab.
From slideplayer.com
Solubility Equilibria ppt download Solubility Equilibria Lab You will test the effects of changing the solution volume and temperature on the solubility. The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in water. There are a number of patterns in the data obtained. We begin our discussion of solubility and complexation equilibria—those associated with the formation of. Solubility Equilibria Lab.
From studylib.net
Solubility Equilibria Equilibrium involving the dissolving Solubility Equilibria Lab The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in water. There are a number of patterns in the data obtained. When a substance such as sodium chloride is added to water, it dissolves, forming an aqueous. You will test the effects of changing the solution volume and temperature on. Solubility Equilibria Lab.
From www.studocu.com
Postlab Template Solubility Equilibria and Temperature (1212K).docx Solubility Equilibria Lab The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of solvent. We begin our discussion of solubility and complexation equilibria—those associated with the formation of. Solubility Equilibria Lab.
From studylib.net
Solubility Equilibria & Complex Ions Know your solubility rules Solubility Equilibria Lab There are a number of patterns in the data obtained. The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of solvent. The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the. Solubility Equilibria Lab.
From slideplayer.com
Aqueous Equilibria Chapter ppt download Solubility Equilibria Lab The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in water. You will test the effects of changing the solution volume and temperature on the solubility. We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. When a substance such as sodium chloride. Solubility Equilibria Lab.
From www.studocu.com
Template Solubility Equilibria CHEM 112 Lab 10 Solubility Solubility Equilibria Lab The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. The solubility of a substance in a solvent is defined as the greatest. Solubility Equilibria Lab.
From www.slideserve.com
PPT Solubility Equilibria PowerPoint Presentation, free download ID Solubility Equilibria Lab The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of solvent. The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in water. We begin our discussion of solubility and complexation equilibria—those associated with the formation of. Solubility Equilibria Lab.
From slidetodoc.com
16 1 Solubility Equilibria Ksp Chapter 15 AcidBase Solubility Equilibria Lab There are a number of patterns in the data obtained. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of solvent. The solubility product constant,. Solubility Equilibria Lab.
From www.studocu.com
Solubility Equilibria Experiment 10 (1 Week) (SOL) Solubility Solubility Equilibria Lab We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. There are a number of patterns in the data obtained. The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. You will test the effects of changing. Solubility Equilibria Lab.
From www.studocu.com
Solubility Equilibria Lab Report Report Solubility Equilibria Solubility Equilibria Lab We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. There are a number of patterns in the data obtained. The equilibrium constant for a dissolution reaction,. Solubility Equilibria Lab.
From www.youtube.com
Solubility Equilibria Complex Ion Equilibria. YouTube Solubility Equilibria Lab The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in water. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. The. Solubility Equilibria Lab.
From www.youtube.com
Solubility Equilibria Lab YouTube Solubility Equilibria Lab The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. The solubility of a substance in a solvent is defined as the greatest amount that will dissolve in equilibrium in a specified volume of solvent. The equilibrium constant for a dissolution reaction, called the. Solubility Equilibria Lab.
From www.youtube.com
Introduction to Solubility Equilibria AP Chem Unit 7, Topic 11a Solubility Equilibria Lab The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. You will test the effects of changing the solution volume and temperature on the solubility. The solubility product constant, kₛₚ, is an equilibrium constant that reflects the extent to which an ionic compound dissolves in water. There are a. Solubility Equilibria Lab.
From www.youtube.com
Solubility Equilibria and Temperature Intro & Theory YouTube Solubility Equilibria Lab The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. You will test the effects of changing the solution volume and temperature on the solubility. The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of. Solubility Equilibria Lab.
From www.studocu.com
CHEM112Solubility Equilibria Lab Solubility Equilibria Lab Purpose Solubility Equilibria Lab There are a number of patterns in the data obtained. We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. The solubility product constant, kₛₚ, is an equilibrium constant that reflects the. Solubility Equilibria Lab.
From scienceinfo.com
Solubility Equilibrium Solubility Equilibria Lab We begin our discussion of solubility and complexation equilibria—those associated with the formation of complex ions—by developing. The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. You will test the effects of changing the solution volume and temperature on the solubility. The amount of salt that must be. Solubility Equilibria Lab.
From studylib.net
Chapter 17 Solubility & Complex Ion Equilibria Solubility Equilibria Lab When a substance such as sodium chloride is added to water, it dissolves, forming an aqueous. There are a number of patterns in the data obtained. The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. The equilibrium constant for a dissolution reaction, called. Solubility Equilibria Lab.
From slideplayer.com
Aqueous Equilibria Chapter ppt download Solubility Equilibria Lab The equilibrium constant for a dissolution reaction, called the solubility product (ksp), is a measure of the solubility of a compound. The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. You will test the effects of changing the solution volume and temperature on. Solubility Equilibria Lab.