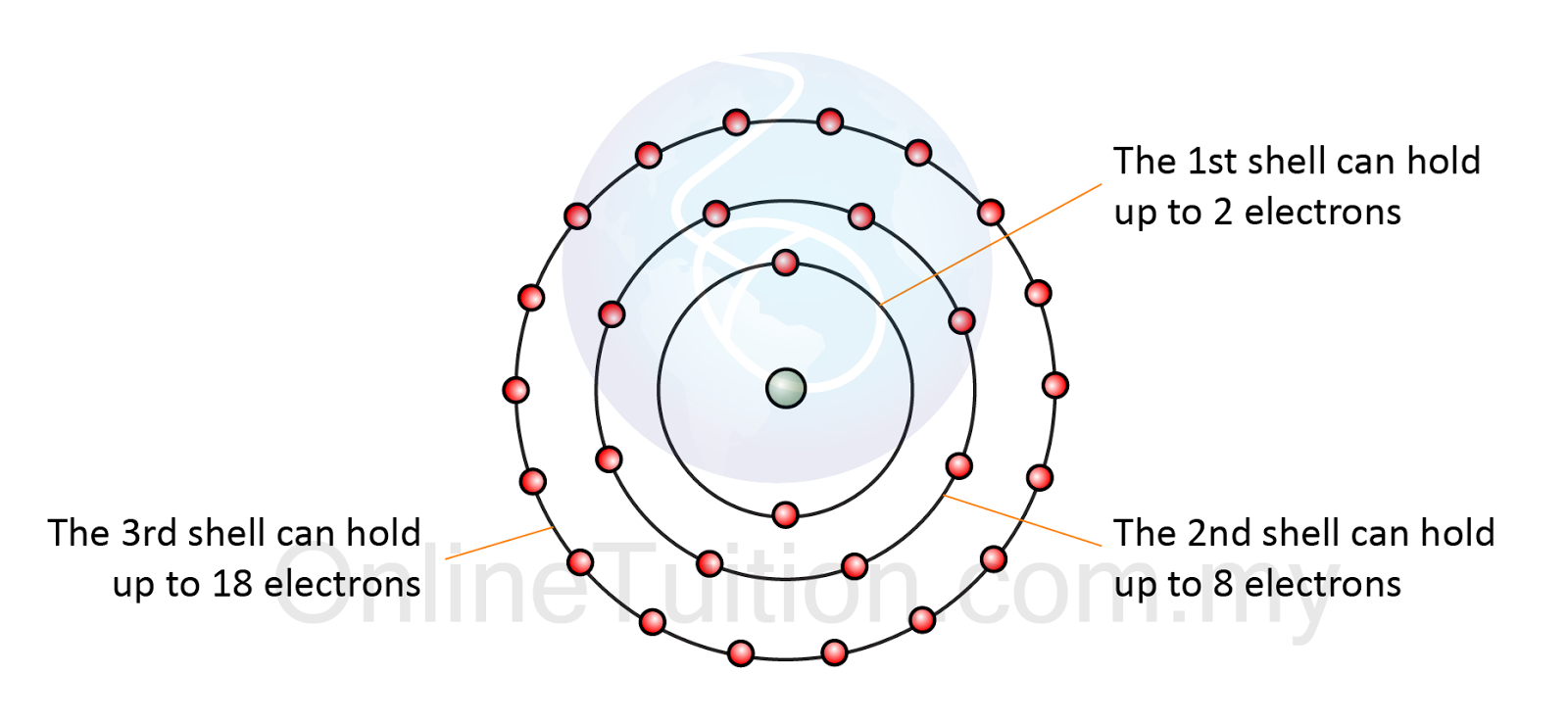
How Does Orbital Electrons Work . This spin can have two. electrons act in some ways as if they were spinning around an axis, somewhat as the earth spins. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. all electrons in an atom want to get as close to the nucleus as possible, but they repel each other. All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. the principal quantum number is one of three quantum numbers used to characterize an orbital. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. How many d orbitals are there in the d subshell? At the first energy level, the only orbital available to electrons is the 1s orbital,. The 2s or 2p orbital? The electron configurations and orbital diagrams of these four elements are: not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). This forces electrons to exist in set regions called. which orbital would the electrons fill first?
from revision.my
electrons act in some ways as if they were spinning around an axis, somewhat as the earth spins. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. The electron configurations and orbital diagrams of these four elements are: the principal quantum number is one of three quantum numbers used to characterize an orbital. which orbital would the electrons fill first? At the first energy level, the only orbital available to electrons is the 1s orbital,. This forces electrons to exist in set regions called. This spin can have two. How many d orbitals are there in the d subshell? All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled.
2.4.1 Electron Arrangement Revision.my
How Does Orbital Electrons Work the principal quantum number is one of three quantum numbers used to characterize an orbital. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. How many d orbitals are there in the d subshell? The 2s or 2p orbital? At the first energy level, the only orbital available to electrons is the 1s orbital,. all electrons in an atom want to get as close to the nucleus as possible, but they repel each other. This forces electrons to exist in set regions called. the principal quantum number is one of three quantum numbers used to characterize an orbital. electrons act in some ways as if they were spinning around an axis, somewhat as the earth spins. The electron configurations and orbital diagrams of these four elements are: All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). This spin can have two. which orbital would the electrons fill first? fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent How Does Orbital Electrons Work All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. This spin can have two. The 2s or 2p orbital? fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. How many d. How Does Orbital Electrons Work.
From www.youtube.com
Orbital Diagrams and Electron Configuration Basic Introduction How Does Orbital Electrons Work electrons act in some ways as if they were spinning around an axis, somewhat as the earth spins. which orbital would the electrons fill first? This spin can have two. the principal quantum number is one of three quantum numbers used to characterize an orbital. This forces electrons to exist in set regions called. How many d. How Does Orbital Electrons Work.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry How Does Orbital Electrons Work the principal quantum number is one of three quantum numbers used to characterize an orbital. which orbital would the electrons fill first? not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. The. How Does Orbital Electrons Work.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps How Does Orbital Electrons Work fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. The 2s or 2p orbital? the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). At the first energy level,. How Does Orbital Electrons Work.
From www.britannica.com
Electron Definition, Mass, & Facts Britannica How Does Orbital Electrons Work not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). This spin can have two. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. all electrons in an atom want to get as close to the nucleus as possible, but they repel each. How Does Orbital Electrons Work.
From www.britannica.com
Orbital Chemistry, Physics & Applications Britannica How Does Orbital Electrons Work This forces electrons to exist in set regions called. At the first energy level, the only orbital available to electrons is the 1s orbital,. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. which orbital would the electrons fill first? all electrons in an atom want to get as close to the. How Does Orbital Electrons Work.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps How Does Orbital Electrons Work the principal quantum number is one of three quantum numbers used to characterize an orbital. all electrons in an atom want to get as close to the nucleus as possible, but they repel each other. electrons act in some ways as if they were spinning around an axis, somewhat as the earth spins. The 2s or 2p. How Does Orbital Electrons Work.
From schematicdatavenin77.z5.web.core.windows.net
Orbital Diagram Definition Chemistry How Does Orbital Electrons Work all electrons in an atom want to get as close to the nucleus as possible, but they repel each other. How many d orbitals are there in the d subshell? electrons act in some ways as if they were spinning around an axis, somewhat as the earth spins. which orbital would the electrons fill first? At the. How Does Orbital Electrons Work.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent How Does Orbital Electrons Work All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. electrons act in some ways as if they were spinning around an axis, somewhat as the earth spins. At the first energy level, the only orbital available. How Does Orbital Electrons Work.
From shaunmwilliams.com
Lecture 7 Presentation How Does Orbital Electrons Work the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. electrons act in some ways as if they were spinning around an axis, somewhat as the earth spins. How many d orbitals are there in the d subshell? At the first energy level, the only orbital available to electrons is. How Does Orbital Electrons Work.
From socratic.org
s,p,d,f Orbitals Chemistry Socratic How Does Orbital Electrons Work the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. At the first energy level, the only orbital available to electrons is the 1s orbital,. The electron configurations and orbital diagrams of these four elements are: fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron.. How Does Orbital Electrons Work.
From wirelibrarystedfast.z21.web.core.windows.net
How To Draw An Orbital Diagram How Does Orbital Electrons Work This forces electrons to exist in set regions called. all electrons in an atom want to get as close to the nucleus as possible, but they repel each other. The electron configurations and orbital diagrams of these four elements are: The 2s or 2p orbital? At the first energy level, the only orbital available to electrons is the 1s. How Does Orbital Electrons Work.
From guidelibneglection.z4.web.core.windows.net
Molecular Orbital Diagram Practice How Does Orbital Electrons Work At the first energy level, the only orbital available to electrons is the 1s orbital,. This spin can have two. This forces electrons to exist in set regions called. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. The 2s or 2p orbital? not all electrons inhabit s orbitals (in fact, very few. How Does Orbital Electrons Work.
From courses.lumenlearning.com
Electrons Biology for Majors I How Does Orbital Electrons Work All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). which orbital would the electrons fill first? the electrons occupying. How Does Orbital Electrons Work.
From www.sciencefacts.net
Atomic Orbital Definition, Types, Shapes, and Diagram How Does Orbital Electrons Work the principal quantum number is one of three quantum numbers used to characterize an orbital. all electrons in an atom want to get as close to the nucleus as possible, but they repel each other. This forces electrons to exist in set regions called. How many d orbitals are there in the d subshell? This spin can have. How Does Orbital Electrons Work.
From chemwiki.ucdavis.edu
Electronic Orbitals Chemwiki How Does Orbital Electrons Work How many d orbitals are there in the d subshell? which orbital would the electrons fill first? the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. This forces electrons to exist in set regions. How Does Orbital Electrons Work.
From chem.libretexts.org
11.5 Molecular Orbital Theory Chemistry LibreTexts How Does Orbital Electrons Work the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. the principal quantum number is one of three quantum numbers used to characterize an orbital. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. not all electrons inhabit s orbitals (in fact, very. How Does Orbital Electrons Work.
From www.youtube.com
Orbitals, the Basics Atomic Orbital Tutorial — probability, shapes How Does Orbital Electrons Work not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). How many d orbitals are there in the d subshell? The electron configurations and orbital diagrams of these four elements are: The 2s or 2p orbital? which orbital would the electrons fill first? fluorine (atomic number 9) has only one 2 p. How Does Orbital Electrons Work.
From polizhuge.weebly.com
Atomic orbitals explained polizhuge How Does Orbital Electrons Work All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). The 2s or 2p orbital? How many d orbitals are there in. How Does Orbital Electrons Work.
From www.expii.com
Shapes of Atomic Orbitals — Overview & Examples Expii How Does Orbital Electrons Work This spin can have two. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. the electrons occupying the outermost shell orbital(s). How Does Orbital Electrons Work.
From polizhuge.weebly.com
Atomic orbitals explained polizhuge How Does Orbital Electrons Work the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. all electrons in an atom want to get as close to the nucleus as possible, but they repel each other. This spin can have two. electrons act in some ways as if they were spinning around an axis, somewhat. How Does Orbital Electrons Work.
From mavink.com
Electron Configuration Chart With Orbitals How Does Orbital Electrons Work electrons act in some ways as if they were spinning around an axis, somewhat as the earth spins. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the. How Does Orbital Electrons Work.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps How Does Orbital Electrons Work the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. This forces electrons to exist in set regions called. This spin can have two. the principal quantum number is one of three quantum numbers used to characterize an orbital. The electron configurations and orbital diagrams of these four elements are:. How Does Orbital Electrons Work.
From gzscienceclassonline.weebly.com
1. Electron Configuration How Does Orbital Electrons Work How many d orbitals are there in the d subshell? not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). which orbital would the electrons fill first? All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the. How Does Orbital Electrons Work.
From guweb2.gonzaga.edu
CHEM 101 Lecture 7 How Does Orbital Electrons Work fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. The electron configurations and orbital diagrams of these four elements are: the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. all electrons in an atom want to get as close to the nucleus as. How Does Orbital Electrons Work.
From www.vedantu.com
Distribution of Electrons in Different Orbits/Shells Learn Important How Does Orbital Electrons Work which orbital would the electrons fill first? not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. the electrons occupying. How Does Orbital Electrons Work.
From saylordotorg.github.io
Atomic Orbitals and Their Energies How Does Orbital Electrons Work All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. This spin can have two. How many d orbitals are there in the d subshell? the principal quantum number is one of three quantum numbers used to. How Does Orbital Electrons Work.
From xaktly.com
Electrons How Does Orbital Electrons Work all electrons in an atom want to get as close to the nucleus as possible, but they repel each other. All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. which orbital would the electrons fill. How Does Orbital Electrons Work.
From guidediagramspumous.z14.web.core.windows.net
Electronic Configuration And Orbital Diagram How Does Orbital Electrons Work All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. which orbital would the electrons fill first? electrons act in some ways as if they were spinning around an axis, somewhat as the earth spins. How. How Does Orbital Electrons Work.
From staff.concord.org
Periodic Table and Bonding Electron Configurations How Does Orbital Electrons Work How many d orbitals are there in the d subshell? The 2s or 2p orbital? fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. the principal quantum number is one of three quantum numbers used to characterize an orbital. all electrons in an atom want to get as close to the nucleus. How Does Orbital Electrons Work.
From www.thoughtco.com
Electron Configuration Chart How Does Orbital Electrons Work This forces electrons to exist in set regions called. the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). This spin can have two. At the first energy level, the only orbital available to electrons. How Does Orbital Electrons Work.
From revision.my
2.4.1 Electron Arrangement Revision.my How Does Orbital Electrons Work the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. which orbital would the electrons fill first? How many d orbitals are there in the d subshell? all electrons in an atom want to get as close to the nucleus as possible, but they repel each other. not. How Does Orbital Electrons Work.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent How Does Orbital Electrons Work not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). The 2s or 2p orbital? all electrons in an atom want to get as close to the nucleus as possible, but they repel each other. At the first energy level, the only orbital available to electrons is the 1s orbital,. The electron configurations. How Does Orbital Electrons Work.
From www.slideserve.com
PPT Orbital Diagrams and Electron Configuration PowerPoint How Does Orbital Electrons Work the electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying. the principal quantum number is one of three quantum numbers used to characterize an orbital. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. This forces electrons to exist in set regions called. . How Does Orbital Electrons Work.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps How Does Orbital Electrons Work The 2s or 2p orbital? not all electrons inhabit s orbitals (in fact, very few electrons live in s orbitals). This forces electrons to exist in set regions called. which orbital would the electrons fill first? The electron configurations and orbital diagrams of these four elements are: fluorine (atomic number 9) has only one 2 p orbital. How Does Orbital Electrons Work.