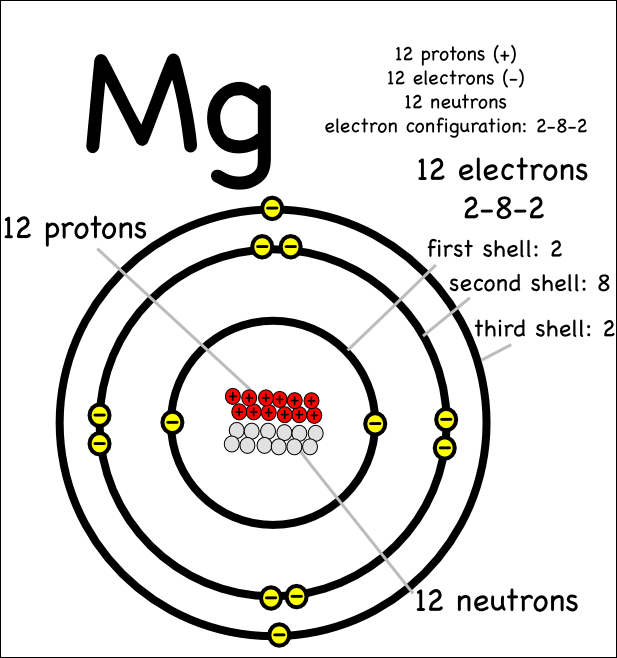
Magnesium Likely Ion . A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. The usual charge of an element is common to its group. The presence of magnesium ions can be detected by the addition of ammonium chloride, ammonium hydroxide and monosodium phosphate to an. There are four ways to find the charge of an element: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Predict which forms an anion, which forms a cation, and the charges of each ion. The symbol for the ion is mg 2+, and it is called a magnesium ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. This electric charge generated on the ion is. 93 rows ionic charge: 93 rows updated on may 07, 2024. This table shows the most common charges for atoms of the chemical elements. Magnesium and nitrogen react to form an ionic compound.
from montessorimuddle.org
There are four ways to find the charge of an element: When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. 93 rows updated on may 07, 2024. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows ionic charge: This electric charge generated on the ion is. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound.
subatomic particles Montessori Muddle
Magnesium Likely Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). There are four ways to find the charge of an element: This table shows the most common charges for atoms of the chemical elements. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The symbol for the ion is mg 2+, and it is called a magnesium ion. 93 rows updated on may 07, 2024. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. This electric charge generated on the ion is. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. The usual charge of an element is common to its group. The presence of magnesium ions can be detected by the addition of ammonium chloride, ammonium hydroxide and monosodium phosphate to an. 93 rows ionic charge: When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge.
From mavink.com
Draw The Orbital Diagram For Magnesium Magnesium Likely Ion Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Magnesium and nitrogen react to form an ionic compound. The symbol for the ion is mg 2+, and it is called a magnesium ion. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Predict which. Magnesium Likely Ion.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Likely Ion Predict which forms an anion, which forms a cation, and the charges of each ion. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. Magnesium Likely Ion.
From www.neuroneeds.com
MAGNESIUM Neuroneeds Magnesium Likely Ion There are four ways to find the charge of an element: 93 rows updated on may 07, 2024. This table shows the most common charges for atoms of the chemical elements. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Magnesium and nitrogen react to form an ionic compound.. Magnesium Likely Ion.
From hongkongholoser.weebly.com
Atomic mass of magnesium hongkongholoser Magnesium Likely Ion 93 rows updated on may 07, 2024. The symbol for the ion is mg 2+, and it is called a magnesium ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Magnesium and nitrogen react to form an ionic compound. When the atom loses. Magnesium Likely Ion.
From www.dreamstime.com
Magnesium chemical element stock vector. Illustration of electron 83098578 Magnesium Likely Ion A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The presence of magnesium ions can be detected by the addition of ammonium. Magnesium Likely Ion.
From www.researchgate.net
Amounts of magnesium ions released from the magnesium ion modified... Download Scientific Diagram Magnesium Likely Ion There are four ways to find the charge of an element: Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. 93 rows ionic charge: The presence of magnesium ions can be detected by the addition of ammonium chloride, ammonium hydroxide and monosodium phosphate to an. The usual charge of an element is common to its. Magnesium Likely Ion.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Likely Ion A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. This electric charge generated on the ion is. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows updated on may 07, 2024. This table shows. Magnesium Likely Ion.
From mungfali.com
Magnesium Comparison Chart Magnesium Likely Ion Magnesium and nitrogen react to form an ionic compound. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Predict which forms an anion, which forms a cation, and the charges of each ion. 93 rows. Magnesium Likely Ion.
From www.numerade.com
SOLVED 38. What ion is likely to be formed by magnesium (Mg) in chemical reactions? Mg Mg2+ Mg Magnesium Likely Ion When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The symbol for the ion is mg 2+, and it is called a magnesium ion. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble. Magnesium Likely Ion.
From www.gauthmath.com
Solved Which nonmetal would an ion of Na1+ MOST LIKELY ioni[algebra] Gauthmath Magnesium Likely Ion The symbol for the ion is mg 2+, and it is called a magnesium ion. This electric charge generated on the ion is. Predict which forms an anion, which forms a cation, and the charges of each ion. 93 rows updated on may 07, 2024. Magnesium and nitrogen react to form an ionic compound. Nitrogen’s position in the periodic table. Magnesium Likely Ion.
From www.difference.wiki
Magnesium Atom vs. Magnesium Ion What’s the Difference? Magnesium Likely Ion 93 rows updated on may 07, 2024. Magnesium and nitrogen react to form an ionic compound. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion. Magnesium Likely Ion.
From www.dreamstime.com
Diagram Representation Of The Element Magnesium Stock Vector Image 59013293 Magnesium Likely Ion The usual charge of an element is common to its group. This electric charge generated on the ion is. 93 rows updated on may 07, 2024. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Nitrogen’s position in the periodic table (group 15) reveals that it is. Magnesium Likely Ion.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Likely Ion The presence of magnesium ions can be detected by the addition of ammonium chloride, ammonium hydroxide and monosodium phosphate to an. There are four ways to find the charge of an element: This electric charge generated on the ion is. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. A magnesium atom must lose two. Magnesium Likely Ion.
From www.transtutors.com
(Solved) Based on the octet rule, magnesium most likely forms a ion. Mg6+... (1 Answer Magnesium Likely Ion A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The usual charge of an element is common to its group. The presence of magnesium ions can be. Magnesium Likely Ion.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Likely Ion The symbol for the ion is mg 2+, and it is called a magnesium ion. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The usual charge of an element is common to its group. A magnesium atom must lose two electrons to have the same number electrons as. Magnesium Likely Ion.
From montessorimuddle.org
subatomic particles Montessori Muddle Magnesium Likely Ion The symbol for the ion is mg 2+, and it is called a magnesium ion. 93 rows updated on may 07, 2024. This table shows the most common charges for atoms of the chemical elements. There are four ways to find the charge of an element: The usual charge of an element is common to its group. The presence of. Magnesium Likely Ion.
From www.vecteezy.com
Magnesium symbol. Chemical element of the periodic table. Vector illustration. 10419830 Vector Magnesium Likely Ion When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The presence of magnesium ions can be detected by the addition of ammonium chloride, ammonium hydroxide and monosodium phosphate to an. When the atom loses or gains one or more electrons, the electric charge is. Magnesium Likely Ion.
From www.numerade.com
SOLVED Write net ionic equation for the reaction that occurs when magnesium sulfide and excess Magnesium Likely Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The symbol for the ion is mg 2+, and it is called a magnesium ion.. Magnesium Likely Ion.
From www.numerade.com
SOLVED most likely ion element symbol of ion type of ion scandium Sc3+ cation anion sulfur s 2 Magnesium Likely Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The presence of magnesium ions can be detected by the addition of ammonium chloride, ammonium hydroxide and monosodium phosphate to an. There are four ways to find the charge of an element: The usual charge of an element is common. Magnesium Likely Ion.
From www.toppr.com
When magnesium makes an ionic bond with oxygen it loses two electrons. What is the charge on the Magnesium Likely Ion The usual charge of an element is common to its group. This table shows the most common charges for atoms of the chemical elements. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Magnesium and nitrogen react to form an ionic compound. Nitrogen’s position in the periodic. Magnesium Likely Ion.
From www.transtutors.com
(Solved) Based on the octet rule, magnesium most likely forms a ion. Mg6+... (1 Answer Magnesium Likely Ion The usual charge of an element is common to its group. 93 rows ionic charge: When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The presence of magnesium ions can be detected by the addition of ammonium chloride, ammonium hydroxide and monosodium phosphate to. Magnesium Likely Ion.
From labpedia.net
Magnesium level (Mg) Magnesium Likely Ion There are four ways to find the charge of an element: The usual charge of an element is common to its group. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. A magnesium atom must lose two electrons to have the same number electrons. Magnesium Likely Ion.
From wholisticmatters.com
Magnesium Blog Part 1 The Importance of Magnesium WholisticMatters Magnesium Likely Ion This table shows the most common charges for atoms of the chemical elements. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. When the atom loses or gains one or more electrons, the. Magnesium Likely Ion.
From www.numerade.com
SOLVED 11. When it forms an ion, is magnesium likely to gain or lose electrons? * 3 points Magnesium Likely Ion This electric charge generated on the ion is. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Predict which forms an anion, which forms. Magnesium Likely Ion.
From www.numerade.com
SOLVED Complete the table Element Predicted charge on its most likely ion 10. Using the metal Magnesium Likely Ion This electric charge generated on the ion is. The presence of magnesium ions can be detected by the addition of ammonium chloride, ammonium hydroxide and monosodium phosphate to an. 93 rows updated on may 07, 2024. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. This table. Magnesium Likely Ion.
From www.researchgate.net
Schematic working principle of sodiumion (a), magnesiumion (b),... Download Scientific Diagram Magnesium Likely Ion The presence of magnesium ions can be detected by the addition of ammonium chloride, ammonium hydroxide and monosodium phosphate to an. Predict which forms an anion, which forms a cation, and the charges of each ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Magnesium Likely Ion.
From www.chegg.com
Solved most likely ion element symbol of ion type of ion Magnesium Likely Ion This electric charge generated on the ion is. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Magnesium and nitrogen react to form an ionic compound. 93 rows ionic charge: When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge,. Magnesium Likely Ion.
From www.numerade.com
SOLVED most likely ion element symbol of ion type of ion 3 + Sc scandium cation anion sulfur Magnesium Likely Ion The symbol for the ion is mg 2+, and it is called a magnesium ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The presence of magnesium ions can be detected by the addition of ammonium chloride, ammonium hydroxide and monosodium phosphate to. Magnesium Likely Ion.
From www.numerade.com
SOLVED most likely ion element symbol of ion type of ion 3 + Sc scandium cation anion sulfur Magnesium Likely Ion Predict which forms an anion, which forms a cation, and the charges of each ion. This table shows the most common charges for atoms of the chemical elements. The symbol for the ion is mg 2+, and it is called a magnesium ion. A magnesium atom must lose two electrons to have the same number electrons as an atom of. Magnesium Likely Ion.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Likely Ion 93 rows updated on may 07, 2024. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge.. Magnesium Likely Ion.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Likely Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. This electric charge generated on the ion is. A magnesium atom must lose two electrons to. Magnesium Likely Ion.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Likely Ion Predict which forms an anion, which forms a cation, and the charges of each ion. The symbol for the ion is mg 2+, and it is called a magnesium ion. 93 rows ionic charge: The usual charge of an element is common to its group. There are four ways to find the charge of an element: Magnesium and nitrogen react. Magnesium Likely Ion.
From www.flexiprep.com
NCERT Class 9 Science Solutions Chapter 3 Atoms and Molecules Part 9 (For CBSE, ICSE, IAS, NET Magnesium Likely Ion Magnesium and nitrogen react to form an ionic compound. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. The usual charge of an element is common to its group. There are four ways to find the charge of an element: When the atom loses or gains one. Magnesium Likely Ion.
From brainly.in
The element magnesium has 2 valence electrons.What is the most likely charge on a magnesium ion Magnesium Likely Ion Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. The symbol for the ion is mg 2+, and it is called a magnesium ion. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. 93 rows updated on may 07, 2024.. Magnesium Likely Ion.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Likely Ion When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. There are four ways to find the charge of an element: The presence of magnesium ions can be detected by the. Magnesium Likely Ion.