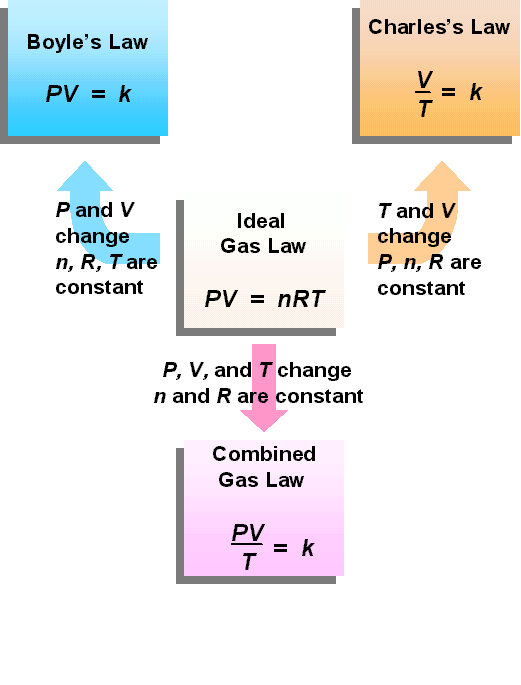
A Pressure Cooker Gas Law . Pressure cookers are designed to cook foods at higher temperatures so the cooking gets done faster! The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a constant. The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. As the pressure inside the cooker increases, the boiling point of water also rises. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a. An example of boyle’s law in action can be seen in a balloon. Okay, what’s the vent stopper have. The key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. This basically states that pressure times volume is equal to the number.
from flowvella.com
Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a. Pressure cookers are designed to cook foods at higher temperatures so the cooking gets done faster! The key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. An example of boyle’s law in action can be seen in a balloon. The ideal gas law is the equation of state of a hypothetical ideal gas. The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. This basically states that pressure times volume is equal to the number. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a constant. Okay, what’s the vent stopper have. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations.
Ideal gas law Screen 5 on FlowVella Presentation Software for Mac
A Pressure Cooker Gas Law Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a. As the pressure inside the cooker increases, the boiling point of water also rises. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a constant. This basically states that pressure times volume is equal to the number. The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. Okay, what’s the vent stopper have. The key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. Pressure cookers are designed to cook foods at higher temperatures so the cooking gets done faster! An example of boyle’s law in action can be seen in a balloon.
From www.pinterest.com
Pressure Cooker Gas Law Pressure Cooker Physics Explained Best A Pressure Cooker Gas Law Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a constant. The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. Okay, what’s the vent stopper have. The ideal gas law is the equation of. A Pressure Cooker Gas Law.
From www.youtube.com
Combined Gas Law Pressure, Volume and Temperature Straight Science A Pressure Cooker Gas Law Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a constant. The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. As the pressure inside the cooker increases, the boiling point of water also rises.. A Pressure Cooker Gas Law.
From www.alamy.com
This stock photo depicts a visually appealing diagram illustrating the A Pressure Cooker Gas Law The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. Pressure cookers are designed to cook foods at higher temperatures so the cooking gets done faster! As the pressure inside the cooker increases, the boiling point of water also rises. This basically states that pressure times volume is equal to the number.. A Pressure Cooker Gas Law.
From fineartamerica.com
Gay Lussac's Pressuretemperature Gas Law Photograph by Science Photo A Pressure Cooker Gas Law The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. As the pressure inside the cooker increases, the boiling point of water also rises. This basically states that pressure times volume is equal to the number. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas,. A Pressure Cooker Gas Law.
From sciencenotes.org
Dalton's Law of Partial Pressure Definition and Examples A Pressure Cooker Gas Law This basically states that pressure times volume is equal to the number. Pressure cookers are designed to cook foods at higher temperatures so the cooking gets done faster! As the pressure inside the cooker increases, the boiling point of water also rises. It is a good approximation to the behavior of many gases under many conditions, although it has several. A Pressure Cooker Gas Law.
From youtube.com
Calculating Gas Pressure Pressure in the Gas Laws YouTube A Pressure Cooker Gas Law Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a constant. An example of boyle’s law in action can be seen in a balloon. This basically states that pressure times volume is equal to the number. The ideal gas law is the equation. A Pressure Cooker Gas Law.
From www.youtube.com
Pressure cookerWorking principleGayLussac's Law YouTube A Pressure Cooker Gas Law The key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. Pressure cookers. A Pressure Cooker Gas Law.
From socratic.org
If you want to study the relationship between temperature and pressure A Pressure Cooker Gas Law Pressure cookers are designed to cook foods at higher temperatures so the cooking gets done faster! The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. Okay, what’s the vent stopper have. An. A Pressure Cooker Gas Law.
From education-portal.com
GayLussac's Law Gas Pressure and Temperature Relationship Video A Pressure Cooker Gas Law It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas,. A Pressure Cooker Gas Law.
From ovenspot.com
Pressure Cooker Gas Law Pressure Cooker Physics Explained A Pressure Cooker Gas Law Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a constant. Okay, what’s the vent stopper have. The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. It is a good approximation to the behavior. A Pressure Cooker Gas Law.
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal A Pressure Cooker Gas Law An example of boyle’s law in action can be seen in a balloon. Okay, what’s the vent stopper have. The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of. A Pressure Cooker Gas Law.
From owlcation.com
The Theories and Behavior of Gas Owlcation A Pressure Cooker Gas Law It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. As the pressure inside the cooker increases, the boiling point of water also rises. Okay, what’s the vent stopper have. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of. A Pressure Cooker Gas Law.
From chemistryguru.com.sg
Ideal Gas Law and Applications A Pressure Cooker Gas Law Okay, what’s the vent stopper have. Pressure cookers are designed to cook foods at higher temperatures so the cooking gets done faster! An example of boyle’s law in action can be seen in a balloon. The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. It is a good approximation to the. A Pressure Cooker Gas Law.
From stock.adobe.com
Gay Lussac Law Infographic Diagram example of pressure cooker A Pressure Cooker Gas Law The key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. This basically states that pressure times volume is equal to the number. An example of boyle’s law in action can be seen in a balloon. It is a good approximation to the behavior of many. A Pressure Cooker Gas Law.
From www.grc.nasa.gov
Boyle's Law A Pressure Cooker Gas Law Okay, what’s the vent stopper have. The key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. As the pressure inside the cooker increases, the boiling point of water also rises. An example of boyle’s law in action can be seen in a balloon. Mathematically, boyle’s. A Pressure Cooker Gas Law.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas A Pressure Cooker Gas Law The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. An example of boyle’s law in action can be seen in a balloon. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of. A Pressure Cooker Gas Law.
From ovenspot.com
Pressure Cooker Gas Law Pressure Cooker Physics Explained A Pressure Cooker Gas Law It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a. Pressure cookers are designed to cook foods at higher temperatures so the cooking gets. A Pressure Cooker Gas Law.
From exosufsdu.blob.core.windows.net
What Gas Law Does A Pressure Cooker Use at Tabitha Newkirk blog A Pressure Cooker Gas Law Pressure cookers are designed to cook foods at higher temperatures so the cooking gets done faster! Okay, what’s the vent stopper have. This basically states that pressure times volume is equal to the number. The ideal gas law is the equation of state of a hypothetical ideal gas. Mathematically, boyle’s law can be written as pv=k, where p is the. A Pressure Cooker Gas Law.
From www.numerade.com
SOLVED 5. The weight on the pressure cooker releases air, lowering the A Pressure Cooker Gas Law Okay, what’s the vent stopper have. The ideal gas law is the equation of state of a hypothetical ideal gas. As the pressure inside the cooker increases, the boiling point of water also rises. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is. A Pressure Cooker Gas Law.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net A Pressure Cooker Gas Law The ideal gas law is the equation of state of a hypothetical ideal gas. An example of boyle’s law in action can be seen in a balloon. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a constant. Okay, what’s the vent stopper. A Pressure Cooker Gas Law.
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock A Pressure Cooker Gas Law The ideal gas law is the equation of state of a hypothetical ideal gas. Pressure cookers are designed to cook foods at higher temperatures so the cooking gets done faster! As the pressure inside the cooker increases, the boiling point of water also rises. The pressure cooker works on the principle of ideal gas law or combined gas law, pv. A Pressure Cooker Gas Law.
From www.youtube.com
11.6 The Combined Gas Law Pressure, Volume, & Temperature YouTube A Pressure Cooker Gas Law An example of boyle’s law in action can be seen in a balloon. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a constant. The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation. A Pressure Cooker Gas Law.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii A Pressure Cooker Gas Law The key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. This basically states that pressure times volume is equal to the number. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. An example of boyle’s. A Pressure Cooker Gas Law.
From mungfali.com
Boyle's Law Chart A Pressure Cooker Gas Law Okay, what’s the vent stopper have. The key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a. It is. A Pressure Cooker Gas Law.
From www.britannica.com
Pressure Definition, Measurement, & Types Britannica A Pressure Cooker Gas Law It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. As the pressure inside the cooker increases, the boiling point of water also rises. Okay, what’s the vent stopper have. Pressure cookers are designed to cook foods at higher temperatures so the cooking gets done faster! Mathematically, boyle’s law can be. A Pressure Cooker Gas Law.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint A Pressure Cooker Gas Law This basically states that pressure times volume is equal to the number. The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. An example of boyle’s law in action can be seen in a balloon. Okay, what’s the vent stopper have. The ideal gas law is the equation of state of a. A Pressure Cooker Gas Law.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii A Pressure Cooker Gas Law Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a. The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. It is a good approximation to the behavior of many gases under many conditions, although. A Pressure Cooker Gas Law.
From ovenspot.com
Pressure Cooker Gas Law Pressure Cooker Physics Explained A Pressure Cooker Gas Law Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a constant. Okay, what’s the vent stopper have. The key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. An. A Pressure Cooker Gas Law.
From sciencenotes.org
GayLussac's Law Definition, Formula, Examples A Pressure Cooker Gas Law This basically states that pressure times volume is equal to the number. An example of boyle’s law in action can be seen in a balloon. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas,. A Pressure Cooker Gas Law.
From flowvella.com
Ideal gas law Screen 5 on FlowVella Presentation Software for Mac A Pressure Cooker Gas Law Pressure cookers are designed to cook foods at higher temperatures so the cooking gets done faster! The key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations.. A Pressure Cooker Gas Law.
From socratic.org
A sample of a gas has a volume of 2.0 liters at a pressure of 1.0 A Pressure Cooker Gas Law An example of boyle’s law in action can be seen in a balloon. The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. The key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. As the pressure inside. A Pressure Cooker Gas Law.
From stravaganzastravaganza.blogspot.com
S T R A V A G A N Z A HISTORY OF PRESSURE COOKER A Pressure Cooker Gas Law It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a constant. Okay, what’s the vent stopper have. The pressure cooker works on the principle. A Pressure Cooker Gas Law.
From www.youtube.com
PVM mRT Ideal Gas law 2 YouTube A Pressure Cooker Gas Law Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a constant. Mathematically, boyle’s law can be written as pv=k, where p is the pressure of the gas, v is the volume of the gas, and k is a. This basically states that pressure. A Pressure Cooker Gas Law.
From exosufsdu.blob.core.windows.net
What Gas Law Does A Pressure Cooker Use at Tabitha Newkirk blog A Pressure Cooker Gas Law As the pressure inside the cooker increases, the boiling point of water also rises. The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. An example of boyle’s law in action can be seen in a balloon. The ideal gas law is the equation of state of a hypothetical ideal gas. This. A Pressure Cooker Gas Law.
From chemistryguru.com.sg
Ideal Gas Law and Applications A Pressure Cooker Gas Law The pressure cooker works on the principle of ideal gas law or combined gas law, pv = nrt. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. The key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law. A Pressure Cooker Gas Law.