Lead Chloride Decomposition Reaction . It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. In concentrated hydrochloric acid, the chloride. A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. Lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. Determine the class of reaction. A solution of sodium chloride is mixed with a solution of lead (ii) nitrate. Because many lead (ii) compounds are. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances.
from www.chegg.com
This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. A solution of sodium chloride is mixed with a solution of lead (ii) nitrate. Lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. In concentrated hydrochloric acid, the chloride. A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Determine the class of reaction. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. Because many lead (ii) compounds are.
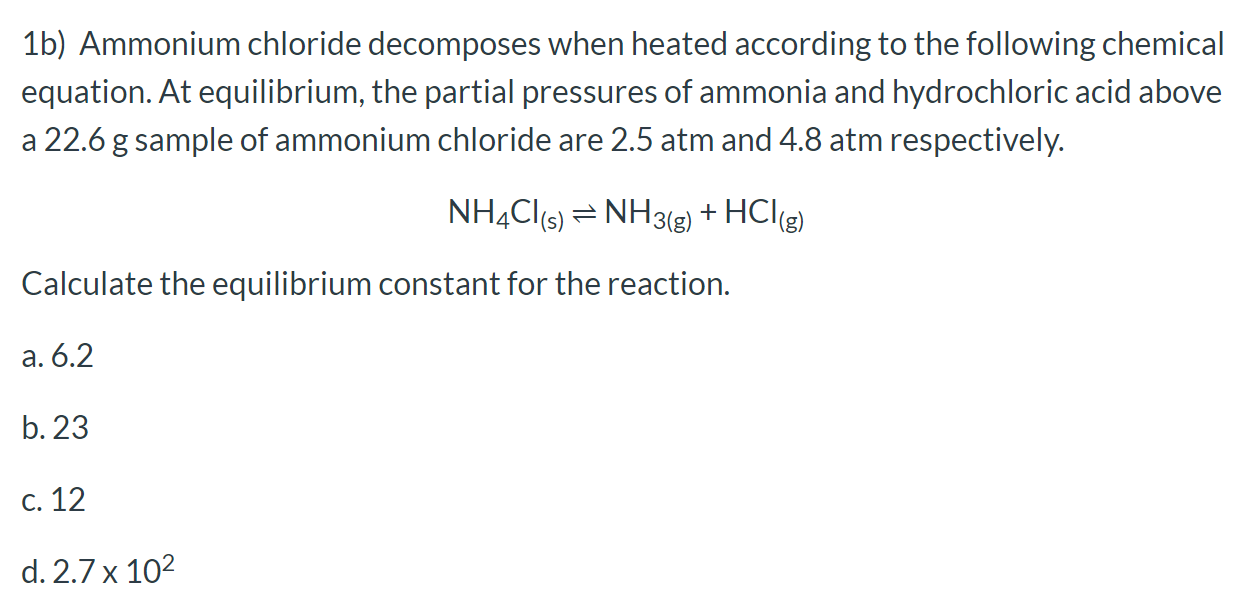
Solved 1b) Ammonium chloride when heated
Lead Chloride Decomposition Reaction A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Because many lead (ii) compounds are. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. In concentrated hydrochloric acid, the chloride. Lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Determine the class of reaction. A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. A solution of sodium chloride is mixed with a solution of lead (ii) nitrate.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Lead Chloride Decomposition Reaction It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. In concentrated hydrochloric acid, the chloride. A solution of sodium chloride is mixed with a solution of lead (ii) nitrate. Because many lead (ii) compounds are. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using. Lead Chloride Decomposition Reaction.
From www.youtube.com
Photolysis Photo Reaction Chemical Reaction and Lead Chloride Decomposition Reaction In concentrated hydrochloric acid, the chloride. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. A solution of sodium chloride is mixed with a solution of lead (ii) nitrate. Lead tetrachloride (lead(iv) chloride). Lead Chloride Decomposition Reaction.
From www.911metallurgist.com
Lead Chloride Electrolysis Lead Chloride Decomposition Reaction Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Determine the class of reaction. In. Lead Chloride Decomposition Reaction.
From www.yaclass.in
reaction — lesson. Science CBSE, Class 10. Lead Chloride Decomposition Reaction A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. In concentrated hydrochloric acid, the chloride. Determine the class of reaction. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions.. Lead Chloride Decomposition Reaction.
From www.numerade.com
SOLVED What kind of chemical reaction does the chemical equation Lead Chloride Decomposition Reaction A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. In concentrated hydrochloric acid, the chloride. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Lead tetrachloride (lead(iv) chloride) the. Lead Chloride Decomposition Reaction.
From chempedia.info
Sodium chloride Big Chemical Encyclopedia Lead Chloride Decomposition Reaction In concentrated hydrochloric acid, the chloride. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one.. Lead Chloride Decomposition Reaction.
From chemicaltweak.com
Types Of Reaction Definition With Examples Equations Lead Chloride Decomposition Reaction A solution of sodium chloride is mixed with a solution of lead (ii) nitrate. A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. Lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions.. Lead Chloride Decomposition Reaction.
From mungfali.com
Electrolysis Of Sodium Chloride Diagram Lead Chloride Decomposition Reaction In concentrated hydrochloric acid, the chloride. Because many lead (ii) compounds are. Lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. A solution. Lead Chloride Decomposition Reaction.
From www.vedantu.com
Reaction Types and Classification of Reaction Lead Chloride Decomposition Reaction This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. In concentrated hydrochloric acid, the chloride. Determine the class. Lead Chloride Decomposition Reaction.
From www.sliderbase.com
Reaction Types Presentation Chemistry Lead Chloride Decomposition Reaction It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. In concentrated hydrochloric acid, the chloride. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances.. Lead Chloride Decomposition Reaction.
From www.worksheetsplanet.com
Reaction Characteristics Lead Chloride Decomposition Reaction It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. In concentrated hydrochloric acid, the chloride. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. Lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. A solution of. Lead Chloride Decomposition Reaction.
From slidetodoc.com
Chemical reactions and chemical equations Chemical reactions are Lead Chloride Decomposition Reaction Determine the class of reaction. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. A solution of sodium chloride is mixed with a solution of lead (ii) nitrate. This page looks at the. Lead Chloride Decomposition Reaction.
From www.chemistrylearner.com
Reaction Definition, Examples, & Applications Lead Chloride Decomposition Reaction A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. Because many lead (ii) compounds are. In concentrated hydrochloric acid, the chloride. Determine the class of reaction. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Lead(ii) is precipitated by chloride, bromide and. Lead Chloride Decomposition Reaction.
From www.expii.com
Reactions — Definition & Examples Expii Lead Chloride Decomposition Reaction This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Determine the class of reaction. A. Lead Chloride Decomposition Reaction.
From chem.libretexts.org
17.7 Electrolysis Chemistry LibreTexts Lead Chloride Decomposition Reaction Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Lead(ii) is precipitated by chloride, bromide. Lead Chloride Decomposition Reaction.
From slidetodoc.com
Chemical reactions and chemical equations Chemical reactions are Lead Chloride Decomposition Reaction Because many lead (ii) compounds are. Determine the class of reaction. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. Lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. A solution of. Lead Chloride Decomposition Reaction.
From fr.dreamstime.com
Diagramme Infographique De Réaction De Illustration de Lead Chloride Decomposition Reaction This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. In concentrated hydrochloric acid, the chloride. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. Lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. A precipitate of. Lead Chloride Decomposition Reaction.
From www.youtube.com
reaction of lead nitrate class 10 of lead Lead Chloride Decomposition Reaction A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. Determine the class of reaction. Lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. In concentrated hydrochloric acid, the chloride. A solution of. Lead Chloride Decomposition Reaction.
From www.slideshare.net
reaction Lead Chloride Decomposition Reaction In concentrated hydrochloric acid, the chloride. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. Determine the class of reaction. A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate.. Lead Chloride Decomposition Reaction.
From www.teachoo.com
Case Based MCQ The following diagram displays a chemical reaction Lead Chloride Decomposition Reaction Lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. A precipitate of lead (ii) chloride results, leaving a. Lead Chloride Decomposition Reaction.
From www.aakash.ac.in
reaction Definition, Classification, Uses and Importance Lead Chloride Decomposition Reaction A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. In concentrated hydrochloric acid, the chloride. A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. This page looks at the. Lead Chloride Decomposition Reaction.
From scienceinfo.com
Reaction 3 Important Types, Uses, Examples Lead Chloride Decomposition Reaction Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. A solution of sodium chloride is mixed with a solution of lead (ii) nitrate. Because many lead (ii) compounds are. In concentrated hydrochloric acid,. Lead Chloride Decomposition Reaction.
From www.youtube.com
Equation for PbCl2 + H2O Lead (II) chloride + Water YouTube Lead Chloride Decomposition Reaction Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. This page looks at the formation of some insoluble lead(ii) compounds. Lead Chloride Decomposition Reaction.
From fphoto.photoshelter.com
science chemistry precipitation reaction lead (ii) chloride Lead Chloride Decomposition Reaction Determine the class of reaction. Because many lead (ii) compounds are. A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. A solution of sodium chloride is mixed with a solution of lead (ii) nitrate. In concentrated hydrochloric acid, the chloride. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using. Lead Chloride Decomposition Reaction.
From www.studocu.com
Simple CHEMICAL REACTION A reaction or Lead Chloride Decomposition Reaction Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. In concentrated hydrochloric acid, the chloride. Lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. Because many lead (ii) compounds are. A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. A decomposition. Lead Chloride Decomposition Reaction.
From encyclopedia.pub
Compounds of Lead Encyclopedia MDPI Lead Chloride Decomposition Reaction In concentrated hydrochloric acid, the chloride. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. A solution of sodium chloride is mixed with a solution of lead (ii) nitrate. Lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. Determine the class of reaction. A precipitate. Lead Chloride Decomposition Reaction.
From www.youtube.com
of lead nitrate ,Thermal reaction YouTube Lead Chloride Decomposition Reaction This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. A solution of sodium chloride is mixed with a solution of lead (ii) nitrate. Lead(ii) is precipitated by chloride, bromide and iodide ions under. Lead Chloride Decomposition Reaction.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Reaction for the Lead Chloride Decomposition Reaction A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. A solution of sodium chloride is mixed with a solution of lead (ii) nitrate. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and. Lead Chloride Decomposition Reaction.
From www.vedantu.com
Reaction Types and Classification of Reaction Lead Chloride Decomposition Reaction Determine the class of reaction. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. In concentrated hydrochloric acid, the chloride. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. A solution of sodium chloride is mixed with a solution of lead (ii). Lead Chloride Decomposition Reaction.
From www.researchgate.net
Mechanism of poly(vinyl chloride) photodegradation. Download Lead Chloride Decomposition Reaction It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. In concentrated hydrochloric acid, the chloride. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one.. Lead Chloride Decomposition Reaction.
From sciencenotes.org
What Is a Reaction? Definition and Examples Lead Chloride Decomposition Reaction A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. Determine the class of reaction. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. This page looks at the formation. Lead Chloride Decomposition Reaction.
From www.numerade.com
The thermal of nitryl chloride, NO2 Cl, 2 NO2 Cl(g) 2 NO2 Lead Chloride Decomposition Reaction It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. Lead(ii) is precipitated by chloride, bromide and iodide ions under cold. Lead Chloride Decomposition Reaction.
From www.chegg.com
Solved 1b) Ammonium chloride when heated Lead Chloride Decomposition Reaction Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Determine the class of reaction. A. Lead Chloride Decomposition Reaction.
From www.researchgate.net
Equilibrium diagram of chloride reaction (a) Al−Cl Lead Chloride Decomposition Reaction In concentrated hydrochloric acid, the chloride. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances.. Lead Chloride Decomposition Reaction.
From slideplayer.com
Types of Chemical Reactions ppt download Lead Chloride Decomposition Reaction It describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Lead tetrachloride (lead(iv) chloride) the reaction of lead(iv) chloride with water is just like the silicon tetrachloride one. A precipitate of lead (ii) chloride results, leaving a solution of sodium nitrated. A solution of sodium chloride is mixed with a solution of. Lead Chloride Decomposition Reaction.