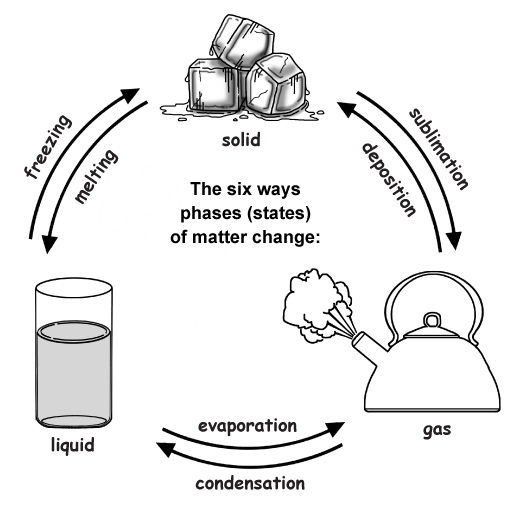
Change Solid Becomes Liquid . Phase transitions play an important theoretical and practical role in the study. changes of state between solid and liquid. In melting (or “ fusion ”), a solid turns into a liquid; The opposite process is condensation. solve calorimetry problems involving phase changes. when the gas becomes a liquid, however, the volume actually decreases precipitously at the liquefaction point. under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a. solve calorimetry problems involving phase changes. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. In evaporation, a liquid turns into a gas; The opposite process is freezing. Remember that particles in a solid are fixed in position and although they can't move around, they are. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Phase transitions play an important theoretical and practical role in the study of heat flow.
from www.exploringnature.org
The opposite process is condensation. The opposite process is freezing. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Phase transitions play an important theoretical and practical role in the study of heat flow. solve calorimetry problems involving phase changes. under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. In evaporation, a liquid turns into a gas; solve calorimetry problems involving phase changes. In melting (or “ fusion ”), a solid turns into a liquid;
Phases of Matter Gas, Liquids, Solids
Change Solid Becomes Liquid Remember that particles in a solid are fixed in position and although they can't move around, they are. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Phase transitions play an important theoretical and practical role in the study. Phase transitions play an important theoretical and practical role in the study of heat flow. The opposite process is condensation. The opposite process is freezing. In evaporation, a liquid turns into a gas; solve calorimetry problems involving phase changes. when the gas becomes a liquid, however, the volume actually decreases precipitously at the liquefaction point. Remember that particles in a solid are fixed in position and although they can't move around, they are. In melting (or “ fusion ”), a solid turns into a liquid; changes of state between solid and liquid. solve calorimetry problems involving phase changes. under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure.
From www.ase.org.uk
Solids, liquids and gases Change Solid Becomes Liquid In evaporation, a liquid turns into a gas; solve calorimetry problems involving phase changes. Phase transitions play an important theoretical and practical role in the study of heat flow. under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a. Every element and substance can transition from one. Change Solid Becomes Liquid.
From slideplayer.com
Matter and the Changes it undergoes ppt download Change Solid Becomes Liquid The opposite process is condensation. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. solve calorimetry problems involving phase changes. Phase transitions play an important theoretical and practical role in the study of heat flow. In evaporation, a liquid turns into a gas; Remember that particles in a solid. Change Solid Becomes Liquid.
From schematiclibmammees88.z22.web.core.windows.net
Solid Liquid Phase Diagram Change Solid Becomes Liquid solve calorimetry problems involving phase changes. when the gas becomes a liquid, however, the volume actually decreases precipitously at the liquefaction point. solve calorimetry problems involving phase changes. changes of state between solid and liquid. In melting (or “ fusion ”), a solid turns into a liquid; Remember that particles in a solid are fixed in. Change Solid Becomes Liquid.
From exotjwucf.blob.core.windows.net
Solid Change To Liquid Example at Robert Vance blog Change Solid Becomes Liquid Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. In melting (or “ fusion ”), a solid turns into a liquid; The opposite process is condensation. changes of state between solid and liquid. Phase transitions play an important theoretical and practical role in the study. The opposite process is. Change Solid Becomes Liquid.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Change Solid Becomes Liquid The opposite process is freezing. changes of state between solid and liquid. Phase transitions play an important theoretical and practical role in the study. solve calorimetry problems involving phase changes. The opposite process is condensation. when the gas becomes a liquid, however, the volume actually decreases precipitously at the liquefaction point. In evaporation, a liquid turns into. Change Solid Becomes Liquid.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Change Solid Becomes Liquid Phase transitions play an important theoretical and practical role in the study. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. changes of state between solid and liquid. when the gas becomes a liquid, however, the volume actually decreases precipitously at the liquefaction point. Remember that particles in. Change Solid Becomes Liquid.
From www.alamy.com
Water States of matter Phase. Change of State for Water Diagram Change Solid Becomes Liquid when the gas becomes a liquid, however, the volume actually decreases precipitously at the liquefaction point. changes of state between solid and liquid. In evaporation, a liquid turns into a gas; Remember that particles in a solid are fixed in position and although they can't move around, they are. phase transition is when a substance changes from. Change Solid Becomes Liquid.
From maxim-kwise.blogspot.com
The Process by Which a Gas Changes Into a Liquid Change Solid Becomes Liquid solve calorimetry problems involving phase changes. under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a. The opposite process is condensation. changes of state between solid and liquid. phase transition is when a substance changes from a solid, liquid, or gas state to a different. Change Solid Becomes Liquid.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk Change Solid Becomes Liquid when the gas becomes a liquid, however, the volume actually decreases precipitously at the liquefaction point. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Phase transitions play an important. Change Solid Becomes Liquid.
From studymind.co.uk
Changing State (GCSE Chemistry) Study Mind Change Solid Becomes Liquid Remember that particles in a solid are fixed in position and although they can't move around, they are. Phase transitions play an important theoretical and practical role in the study. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. In melting (or “ fusion ”), a solid turns into a. Change Solid Becomes Liquid.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Change Solid Becomes Liquid Phase transitions play an important theoretical and practical role in the study. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Remember that particles in a solid are fixed in position and although they can't move around, they are. under some circumstances, the solid phase can transition directly to. Change Solid Becomes Liquid.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock Change Solid Becomes Liquid when the gas becomes a liquid, however, the volume actually decreases precipitously at the liquefaction point. solve calorimetry problems involving phase changes. The opposite process is freezing. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. phase transition is when a substance changes from a solid, liquid,. Change Solid Becomes Liquid.
From www.slideserve.com
PPT CHAPTER 2 SOLIDS, LIQUIDS AND GASES PowerPoint Presentation Change Solid Becomes Liquid The opposite process is condensation. when the gas becomes a liquid, however, the volume actually decreases precipitously at the liquefaction point. The opposite process is freezing. under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a. In melting (or “ fusion ”), a solid turns into a. Change Solid Becomes Liquid.
From www.snexplores.org
Explainer What are the different states of matter? Change Solid Becomes Liquid Phase transitions play an important theoretical and practical role in the study of heat flow. In evaporation, a liquid turns into a gas; solve calorimetry problems involving phase changes. The opposite process is condensation. when the gas becomes a liquid, however, the volume actually decreases precipitously at the liquefaction point. In melting (or “ fusion ”), a solid. Change Solid Becomes Liquid.
From dxowmcqql.blob.core.windows.net
Solid Liquid Definition at Rodney Folkes blog Change Solid Becomes Liquid The opposite process is freezing. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. solve calorimetry problems involving phase changes. Remember that particles in a solid are fixed in position and although they can't move around, they are. changes of state between solid and liquid. solve calorimetry. Change Solid Becomes Liquid.
From www.vedantu.com
Which Liquid Solid on Heating Learn Important Terms and Concepts Change Solid Becomes Liquid solve calorimetry problems involving phase changes. under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a. The opposite process is freezing. Phase transitions play an important theoretical and practical role in the study. phase transition is when a substance changes from a solid, liquid, or gas. Change Solid Becomes Liquid.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Change Solid Becomes Liquid The opposite process is condensation. Remember that particles in a solid are fixed in position and although they can't move around, they are. solve calorimetry problems involving phase changes. In melting (or “ fusion ”), a solid turns into a liquid; Phase transitions play an important theoretical and practical role in the study. Phase transitions play an important theoretical. Change Solid Becomes Liquid.
From circuitdbseriatim.z13.web.core.windows.net
Solid Liquid Gas Diagram Change Solid Becomes Liquid solve calorimetry problems involving phase changes. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. The opposite process is freezing. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Phase transitions play an important theoretical and practical role in. Change Solid Becomes Liquid.
From guidelistbaquantising.z13.web.core.windows.net
Venn Diagram For Solids Liquids And Gases Change Solid Becomes Liquid phase transition is when a substance changes from a solid, liquid, or gas state to a different state. under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a. In evaporation, a liquid turns into a gas; solve calorimetry problems involving phase changes. solve calorimetry problems. Change Solid Becomes Liquid.
From www.slideserve.com
PPT Phase Change PowerPoint Presentation, free download ID5735222 Change Solid Becomes Liquid under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a. The opposite process is freezing. solve calorimetry problems involving phase changes. In melting (or “ fusion ”), a solid turns into a liquid; Phase transitions play an important theoretical and practical role in the study of heat. Change Solid Becomes Liquid.
From kids.britannica.com
solid Students Britannica Kids Homework Help Change Solid Becomes Liquid In evaporation, a liquid turns into a gas; changes of state between solid and liquid. when the gas becomes a liquid, however, the volume actually decreases precipitously at the liquefaction point. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Phase transitions play an important theoretical and practical. Change Solid Becomes Liquid.
From courses.lumenlearning.com
Phase Changes Boundless Chemistry Change Solid Becomes Liquid In melting (or “ fusion ”), a solid turns into a liquid; solve calorimetry problems involving phase changes. under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a. changes of state between solid and liquid. The opposite process is condensation. solve calorimetry problems involving phase. Change Solid Becomes Liquid.
From www.techexplorist.com
Elements can be solid and liquid at the same time Change Solid Becomes Liquid In evaporation, a liquid turns into a gas; changes of state between solid and liquid. In melting (or “ fusion ”), a solid turns into a liquid; Phase transitions play an important theoretical and practical role in the study of heat flow. Every element and substance can transition from one phase to another at a specific combination of temperature. Change Solid Becomes Liquid.
From www.vecteezy.com
Three different States of matter solid, liquid and gasuas state. Inter Change Solid Becomes Liquid changes of state between solid and liquid. solve calorimetry problems involving phase changes. Phase transitions play an important theoretical and practical role in the study of heat flow. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. In melting (or “ fusion ”), a solid turns into a. Change Solid Becomes Liquid.
From slideplayer.com
Notes Section 2.2 States of Matter Matter and Energy. ppt download Change Solid Becomes Liquid solve calorimetry problems involving phase changes. Phase transitions play an important theoretical and practical role in the study of heat flow. The opposite process is freezing. Phase transitions play an important theoretical and practical role in the study. under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Change Solid Becomes Liquid.
From www.youtube.com
Changes in Materials when Mixed with other Materials (Part 3 Solid to Change Solid Becomes Liquid Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The opposite process is freezing. under some circumstances, the solid phase can transition directly to the gas phase without going through. Change Solid Becomes Liquid.
From www.slideserve.com
PPT Phase Change PowerPoint Presentation, free download ID5735222 Change Solid Becomes Liquid In evaporation, a liquid turns into a gas; solve calorimetry problems involving phase changes. under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a. when the gas becomes a liquid, however, the volume actually decreases precipitously at the liquefaction point. The opposite process is freezing. Every. Change Solid Becomes Liquid.
From exotjwucf.blob.core.windows.net
Solid Change To Liquid Example at Robert Vance blog Change Solid Becomes Liquid The opposite process is condensation. solve calorimetry problems involving phase changes. solve calorimetry problems involving phase changes. In melting (or “ fusion ”), a solid turns into a liquid; The opposite process is freezing. Phase transitions play an important theoretical and practical role in the study. Every element and substance can transition from one phase to another at. Change Solid Becomes Liquid.
From chem.libretexts.org
Intro to Phases and Intermolecular Forces Chemistry LibreTexts Change Solid Becomes Liquid under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a. changes of state between solid and liquid. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Remember that particles in a solid are fixed in position and. Change Solid Becomes Liquid.
From www.slideserve.com
PPT Molecular Theory States of Matter Phase Changes Change Solid Becomes Liquid In melting (or “ fusion ”), a solid turns into a liquid; Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. The opposite process is freezing. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. when the gas becomes. Change Solid Becomes Liquid.
From www.numerade.com
SOLVED 'Please help me with this homework Complete the following chart Change Solid Becomes Liquid solve calorimetry problems involving phase changes. The opposite process is freezing. Phase transitions play an important theoretical and practical role in the study. Remember that particles in a solid are fixed in position and although they can't move around, they are. In melting (or “ fusion ”), a solid turns into a liquid; In evaporation, a liquid turns into. Change Solid Becomes Liquid.
From www.thoughtco.com
List of Phase Changes Between States of Matter Change Solid Becomes Liquid In melting (or “ fusion ”), a solid turns into a liquid; In evaporation, a liquid turns into a gas; Phase transitions play an important theoretical and practical role in the study. changes of state between solid and liquid. under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase,. Change Solid Becomes Liquid.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Change Solid Becomes Liquid In evaporation, a liquid turns into a gas; phase transition is when a substance changes from a solid, liquid, or gas state to a different state. under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a. Phase transitions play an important theoretical and practical role in the. Change Solid Becomes Liquid.
From dxogzljcw.blob.core.windows.net
Solid Changes To Liquid Is Called at Sharon Smith blog Change Solid Becomes Liquid Phase transitions play an important theoretical and practical role in the study of heat flow. changes of state between solid and liquid. phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can transition from one phase to another at a specific combination of temperature and. Change Solid Becomes Liquid.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Change Solid Becomes Liquid Remember that particles in a solid are fixed in position and although they can't move around, they are. The opposite process is condensation. when the gas becomes a liquid, however, the volume actually decreases precipitously at the liquefaction point. In melting (or “ fusion ”), a solid turns into a liquid; under some circumstances, the solid phase can. Change Solid Becomes Liquid.