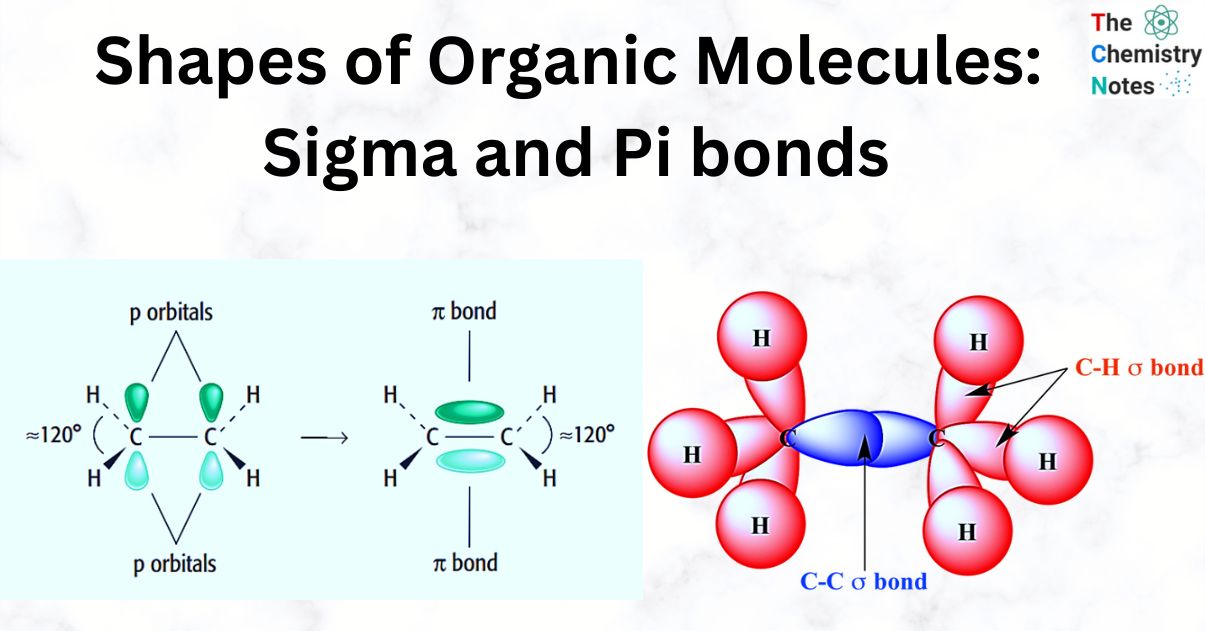
Define Pi Bond Ochem . This plane contains the six atoms and all of the sigma bonds. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple. pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a different atom. explain the local nature of σ and the global nature of π bonds as well as how electrons in p orbitals andπ bonds conjugate. Pi bonds are often written as ‘𝛑 bonds’, where the greek letter ‘𝛑’ refers to the similar symmetry of the pi bond and the p orbital. it is important to train our eye to recognize structural features that have stabilizing effects. Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes the molecule or ion.
from thechemistrynotes.com
the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. This plane contains the six atoms and all of the sigma bonds. Pi bonds are often written as ‘𝛑 bonds’, where the greek letter ‘𝛑’ refers to the similar symmetry of the pi bond and the p orbital. Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes the molecule or ion. explain the local nature of σ and the global nature of π bonds as well as how electrons in p orbitals andπ bonds conjugate. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple. it is important to train our eye to recognize structural features that have stabilizing effects. pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a different atom.
Shapes of Organic Molecules Sigma and Pi bonds
Define Pi Bond Ochem pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a different atom. explain the local nature of σ and the global nature of π bonds as well as how electrons in p orbitals andπ bonds conjugate. pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a different atom. This plane contains the six atoms and all of the sigma bonds. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Pi bonds are often written as ‘𝛑 bonds’, where the greek letter ‘𝛑’ refers to the similar symmetry of the pi bond and the p orbital. Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes the molecule or ion. it is important to train our eye to recognize structural features that have stabilizing effects. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple.
From www.expii.com
Sigma and Pi Bonds — Definition & Overview Expii Define Pi Bond Ochem Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes the molecule or ion. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. . Define Pi Bond Ochem.
From www.chemistrystudent.com
Sigma (δ) and Pi ((π) Bond (ALevel) ChemistryStudent Define Pi Bond Ochem pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a different atom. explain the local nature of σ and the global nature of π bonds as well as how electrons in p orbitals andπ bonds. Define Pi Bond Ochem.
From www.youtube.com
Sigma and Pi Bonds Explained, Basic Introduction, Chemistry YouTube Define Pi Bond Ochem the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. it is important to train our eye to recognize structural features that have stabilizing effects. Alternating single and double bonds create a conjugated pi. Define Pi Bond Ochem.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Pi bond Define Pi Bond Ochem Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes the molecule or ion. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple. This plane contains the six atoms and all of the sigma bonds.. Define Pi Bond Ochem.
From www.vrogue.co
Bonding And Antibonding Pi Orbitals Master Organic Ch vrogue.co Define Pi Bond Ochem Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes the molecule or ion. explain the local nature of σ and the global nature of π bonds as well as how electrons in p orbitals andπ bonds conjugate. This plane contains the six atoms and all of the sigma. Define Pi Bond Ochem.
From dxoniygmg.blob.core.windows.net
Define Pi Bond Class 11 at Gerardo Gerdes blog Define Pi Bond Ochem explain the local nature of σ and the global nature of π bonds as well as how electrons in p orbitals andπ bonds conjugate. This plane contains the six atoms and all of the sigma bonds. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and. Define Pi Bond Ochem.
From www.thoughtco.com
How Do You Define a Pi Bond in Chemistry? Define Pi Bond Ochem Pi bonds are often written as ‘𝛑 bonds’, where the greek letter ‘𝛑’ refers to the similar symmetry of the pi bond and the p orbital. pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a. Define Pi Bond Ochem.
From www.vrogue.co
Sigma And Pi Bonds Definition And Detailed Explanatio vrogue.co Define Pi Bond Ochem Pi bonds are often written as ‘𝛑 bonds’, where the greek letter ‘𝛑’ refers to the similar symmetry of the pi bond and the p orbital. explain the local nature of σ and the global nature of π bonds as well as how electrons in p orbitals andπ bonds conjugate. pi bonds are chemical bonds that are covalent. Define Pi Bond Ochem.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID1951267 Define Pi Bond Ochem it is important to train our eye to recognize structural features that have stabilizing effects. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. pi bonds are chemical bonds that are covalent. Define Pi Bond Ochem.
From circuitwiringnears88.z22.web.core.windows.net
Pi Molecular Orbital Diagram Define Pi Bond Ochem Pi bonds are often written as ‘𝛑 bonds’, where the greek letter ‘𝛑’ refers to the similar symmetry of the pi bond and the p orbital. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the. Define Pi Bond Ochem.
From brilliant.org
Sigma and Pi Bonds Brilliant Math & Science Wiki Define Pi Bond Ochem explain the local nature of σ and the global nature of π bonds as well as how electrons in p orbitals andπ bonds conjugate. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple. Pi bonds are often written as ‘𝛑 bonds’, where the greek. Define Pi Bond Ochem.
From www.youtube.com
Sigma and Pi Bond Symmetry YouTube Define Pi Bond Ochem This plane contains the six atoms and all of the sigma bonds. Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes the molecule or ion. explain the local nature of σ and the global nature of π bonds as well as how electrons in p orbitals andπ bonds. Define Pi Bond Ochem.
From www.youtube.com
Hybridization of Atomic Orbitals Sigma & Pi Bonds Sp, Sp2, Sp3 Define Pi Bond Ochem Pi bonds are often written as ‘𝛑 bonds’, where the greek letter ‘𝛑’ refers to the similar symmetry of the pi bond and the p orbital. it is important to train our eye to recognize structural features that have stabilizing effects. This plane contains the six atoms and all of the sigma bonds. sigma and pi bonds are. Define Pi Bond Ochem.
From ditki.com
Biochemistry Glossary Bonds 3. Pi Bonds Overlap ditki medical Define Pi Bond Ochem it is important to train our eye to recognize structural features that have stabilizing effects. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple. Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes. Define Pi Bond Ochem.
From www.masterorganicchemistry.com
Conjugation And Resonance In Organic Chemistry Define Pi Bond Ochem This plane contains the six atoms and all of the sigma bonds. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. sigma and pi bonds are an aspect of valence bond theory and. Define Pi Bond Ochem.
From thechemistrynotes.com
Shapes of Organic Molecules Sigma and Pi bonds Define Pi Bond Ochem sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. . Define Pi Bond Ochem.
From www.youtube.com
Sigma and Pi bonds Organic Chemistry Made Simple YouTube Define Pi Bond Ochem pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a different atom. explain the local nature of σ and the global nature of π bonds as well as how electrons in p orbitals andπ bonds. Define Pi Bond Ochem.
From www.youtube.com
How To Calculate Sigma And Pi Bond Super Trick to Find Sigma and Pi Define Pi Bond Ochem Pi bonds are often written as ‘𝛑 bonds’, where the greek letter ‘𝛑’ refers to the similar symmetry of the pi bond and the p orbital. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple. the pi bond is the second bond of the. Define Pi Bond Ochem.
From dxoniygmg.blob.core.windows.net
Define Pi Bond Class 11 at Gerardo Gerdes blog Define Pi Bond Ochem Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes the molecule or ion. it is important to train our eye to recognize structural features that have stabilizing effects. This plane contains the six atoms and all of the sigma bonds. sigma and pi bonds are an aspect. Define Pi Bond Ochem.
From www.youtube.com
TRICK CALCULATE PI AND SIGMA BONDS IN A RING ORGANIC CHEMISTRY Define Pi Bond Ochem pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a different atom. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple.. Define Pi Bond Ochem.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Pi bond Define Pi Bond Ochem Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes the molecule or ion. the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Pi. Define Pi Bond Ochem.
From thechemistrynotes.com
Shapes of Organic Molecules Sigma and Pi bonds Define Pi Bond Ochem pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a different atom. it is important to train our eye to recognize structural features that have stabilizing effects. the pi bond is the second bond. Define Pi Bond Ochem.
From www.youtube.com
9.4 Sigma Bonds and Pi Bonds General Chemistry YouTube Define Pi Bond Ochem it is important to train our eye to recognize structural features that have stabilizing effects. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple. explain the local nature of σ and the global nature of π bonds as well as how electrons in. Define Pi Bond Ochem.
From testbook.com
Pi Bonds Know Definition, Formation, Characteristics & Examples Define Pi Bond Ochem pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a different atom. This plane contains the six atoms and all of the sigma bonds. Alternating single and double bonds create a conjugated pi bond system across. Define Pi Bond Ochem.
From slideplayer.com
SUPA Chemistry Sigma and Pi Bonds. ppt download Define Pi Bond Ochem pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a different atom. explain the local nature of σ and the global nature of π bonds as well as how electrons in p orbitals andπ bonds. Define Pi Bond Ochem.
From slideplayer.com
Sigma and Pi Bonding. ppt download Define Pi Bond Ochem explain the local nature of σ and the global nature of π bonds as well as how electrons in p orbitals andπ bonds conjugate. This plane contains the six atoms and all of the sigma bonds. Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes the molecule or. Define Pi Bond Ochem.
From www.youtube.com
How to determine Sigma and Pi Bond Hybridization Organic Chemistry Define Pi Bond Ochem sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple. Pi bonds are often written as ‘𝛑 bonds’, where the greek letter ‘𝛑’ refers to the similar symmetry of the pi bond and the p orbital. pi bonds are chemical bonds that are covalent in. Define Pi Bond Ochem.
From www.masterorganicchemistry.com
Resonance in Organic Chemistry Pi Donors and Pi Donation — Master Define Pi Bond Ochem Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes the molecule or ion. sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple. This plane contains the six atoms and all of the sigma bonds.. Define Pi Bond Ochem.
From www.pearson.com
Draw orbital pictures of the pi bonding in the following compound Define Pi Bond Ochem the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes the molecule or ion. . Define Pi Bond Ochem.
From www.pinterest.com
Pi Bond Pi bond, Chemistry education, Teaching chemistry Define Pi Bond Ochem the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two. Define Pi Bond Ochem.
From www.doubtnut.com
The number of sigmaand pibond in 1butene3yne is Define Pi Bond Ochem the pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Pi bonds are often written as ‘𝛑 bonds’, where the greek letter ‘𝛑’ refers to the similar symmetry of the pi bond and the p. Define Pi Bond Ochem.
From www.difference101.com
Pi vs. Sigma Bond 6 Key Differences, Pros & Cons, Similarities Define Pi Bond Ochem Pi bonds are often written as ‘𝛑 bonds’, where the greek letter ‘𝛑’ refers to the similar symmetry of the pi bond and the p orbital. Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes the molecule or ion. the pi bond is the second bond of the. Define Pi Bond Ochem.
From www.coursehero.com
[Solved] . Draw a resonance structure that places a pi bond in a Define Pi Bond Ochem Pi bonds are often written as ‘𝛑 bonds’, where the greek letter ‘𝛑’ refers to the similar symmetry of the pi bond and the p orbital. explain the local nature of σ and the global nature of π bonds as well as how electrons in p orbitals andπ bonds conjugate. This plane contains the six atoms and all of. Define Pi Bond Ochem.
From www.masterorganicchemistry.com
Conjugation And Resonance In Organic Chemistry Define Pi Bond Ochem pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a different atom. This plane contains the six atoms and all of the sigma bonds. Alternating single and double bonds create a conjugated pi bond system across. Define Pi Bond Ochem.
From byjus.com
A π bond is formed by the overlap of Define Pi Bond Ochem it is important to train our eye to recognize structural features that have stabilizing effects. explain the local nature of σ and the global nature of π bonds as well as how electrons in p orbitals andπ bonds conjugate. Pi bonds are often written as ‘𝛑 bonds’, where the greek letter ‘𝛑’ refers to the similar symmetry of. Define Pi Bond Ochem.