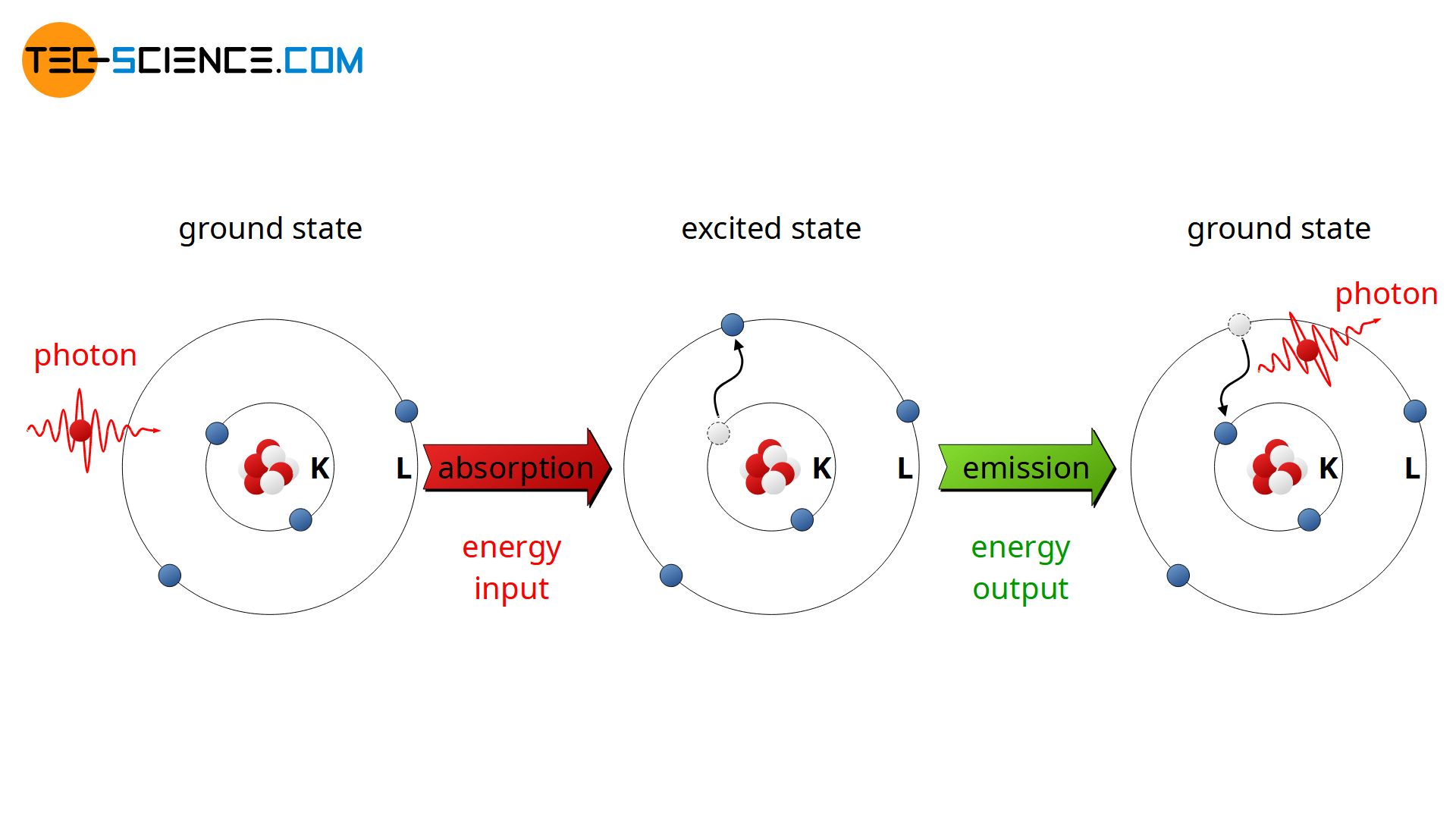
Light Is Absorbed When Electrons . Light absorption is the process in which light is absorbed and converted into energy. When an atom emits light, it decays to a lower energy state; When an electron jumps from a. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. How does light reveal the behavior of electrons in an atom? It's a common understanding that atoms emit or absorb light when the energy of the photons is equal to the the difference in the. Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c, of 2.998 × 10 8 m s −1. When an atom absorbs light, it is excited to a higher energy state. From fi reworks to stars, the color of light is useful in fi nding out what’s in matter.
from www.tec-science.com
When an atom emits light, it decays to a lower energy state; From fi reworks to stars, the color of light is useful in fi nding out what’s in matter. When an atom absorbs light, it is excited to a higher energy state. How does light reveal the behavior of electrons in an atom? Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c, of 2.998 × 10 8 m s −1. Light absorption is the process in which light is absorbed and converted into energy. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. It's a common understanding that atoms emit or absorb light when the energy of the photons is equal to the the difference in the. When an electron jumps from a.
Bohr's atomic model tecscience
Light Is Absorbed When Electrons How does light reveal the behavior of electrons in an atom? When an atom absorbs light, it is excited to a higher energy state. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. It's a common understanding that atoms emit or absorb light when the energy of the photons is equal to the the difference in the. From fi reworks to stars, the color of light is useful in fi nding out what’s in matter. When an electron jumps from a. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. When an atom emits light, it decays to a lower energy state; How does light reveal the behavior of electrons in an atom? Light absorption is the process in which light is absorbed and converted into energy. Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c, of 2.998 × 10 8 m s −1.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Light Is Absorbed When Electrons Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c, of 2.998 × 10 8 m s −1. When an atom emits light, it decays to a lower energy state; Light absorption is the process in which light is absorbed and converted into energy. From fi reworks to stars, the color of light is. Light Is Absorbed When Electrons.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Light Is Absorbed When Electrons It's a common understanding that atoms emit or absorb light when the energy of the photons is equal to the the difference in the. From fi reworks to stars, the color of light is useful in fi nding out what’s in matter. Light absorption is the process in which light is absorbed and converted into energy. Light and other forms. Light Is Absorbed When Electrons.
From raider.pressbooks.pub
Chapter 23 Photosynthesis Lightdependent Reactions Introductory Light Is Absorbed When Electrons From fi reworks to stars, the color of light is useful in fi nding out what’s in matter. When an atom absorbs light, it is excited to a higher energy state. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. Light and other forms of electromagnetic radiation move through a vacuum with a constant. Light Is Absorbed When Electrons.
From www.britannica.com
Compton effect Definition, Formula, & Facts Britannica Light Is Absorbed When Electrons When an atom emits light, it decays to a lower energy state; Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. How does light reveal the behavior of electrons in an atom? From fi reworks to stars, the color of light is useful in fi nding out. Light Is Absorbed When Electrons.
From users.highland.edu
Atomic Spectra and Models of the Atom Light Is Absorbed When Electrons Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. When an electron jumps from a. How does light reveal the behavior of electrons in an atom? It's a common understanding that atoms. Light Is Absorbed When Electrons.
From www.slideserve.com
PPT Modern Atomic Theory Electrons in the Atom Light Is Absorbed When Electrons When an electron jumps from a. Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c, of 2.998 × 10 8 m s −1. Light absorption is the process in which light is absorbed and converted into energy. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their. Light Is Absorbed When Electrons.
From rwu.pressbooks.pub
Chapter 12. Photosynthesis Introduction to Molecular and Cell Biology Light Is Absorbed When Electrons When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c, of 2.998 × 10 8 m s. Light Is Absorbed When Electrons.
From slideplayer.com
ppt download Light Is Absorbed When Electrons Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. When an atom emits light, it decays to a lower energy state; Light absorption is the process in which light is absorbed and converted into energy. How does light reveal the behavior of electrons in an atom? From. Light Is Absorbed When Electrons.
From montessorimuddle.org
Emission Spectra How Atoms Emit and Absorb Light Montessori Muddle Light Is Absorbed When Electrons How does light reveal the behavior of electrons in an atom? When an electron jumps from a. When an atom absorbs light, it is excited to a higher energy state. From fi reworks to stars, the color of light is useful in fi nding out what’s in matter. Light absorption is the process in which light is absorbed and converted. Light Is Absorbed When Electrons.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Light Is Absorbed When Electrons Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. When an electron jumps from a. Light absorption is the process in which light is absorbed and converted into energy. From fi reworks to stars, the color of light is useful in fi nding out what’s in matter.. Light Is Absorbed When Electrons.
From loepetamz.blob.core.windows.net
How Is A Photon Absorbed at Phillip Martinson blog Light Is Absorbed When Electrons It's a common understanding that atoms emit or absorb light when the energy of the photons is equal to the the difference in the. Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c, of 2.998 × 10 8 m s −1. When an atom absorbs light, it is excited to a higher energy. Light Is Absorbed When Electrons.
From www.youtube.com
Electron Energy and Light Spectra YouTube Light Is Absorbed When Electrons How does light reveal the behavior of electrons in an atom? When an atom absorbs light, it is excited to a higher energy state. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. It's a common understanding that atoms emit or absorb light when the energy of. Light Is Absorbed When Electrons.
From www.pinterest.com
Light and photosynthetic pigments The lightdependent reactions Light Is Absorbed When Electrons Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. From fi reworks to stars, the color of light is useful in fi nding out what’s in matter. When an atom emits light,. Light Is Absorbed When Electrons.
From www.tec-science.com
Bohr's atomic model tecscience Light Is Absorbed When Electrons Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c, of 2.998 × 10 8 m s −1. When an atom absorbs light, it is excited to a higher energy state. It's a common understanding that atoms emit or absorb light when the energy of the photons is equal to the the difference in. Light Is Absorbed When Electrons.
From www.slideserve.com
PPT 4.1Capturing Solar Energy Light Dependent Reactions PowerPoint Light Is Absorbed When Electrons It's a common understanding that atoms emit or absorb light when the energy of the photons is equal to the the difference in the. From fi reworks to stars, the color of light is useful in fi nding out what’s in matter. When an atom absorbs light, it is excited to a higher energy state. When electrons absorb energy, they. Light Is Absorbed When Electrons.
From montessorimuddle.org
Emission Spectra How Atoms Emit and Absorb Light Montessori Muddle Light Is Absorbed When Electrons When an atom emits light, it decays to a lower energy state; How does light reveal the behavior of electrons in an atom? Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. From fi reworks to stars, the color of light is useful in fi nding out. Light Is Absorbed When Electrons.
From www.jobilize.com
What happens when a compound absorbs a photon of light By OpenStax Light Is Absorbed When Electrons Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. It's a common understanding that atoms emit or absorb light when the energy of the photons is equal to the the difference in the. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which. Light Is Absorbed When Electrons.
From www.slideserve.com
PPT Chapter 5 Light PowerPoint Presentation, free download ID2768132 Light Is Absorbed When Electrons Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Light absorption is the process in which light is absorbed and converted into energy. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. When an atom absorbs light, it is excited to. Light Is Absorbed When Electrons.
From www.slideserve.com
PPT Chapter 10 Photosynthesis PowerPoint Presentation, free download Light Is Absorbed When Electrons When an atom emits light, it decays to a lower energy state; When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. When an atom absorbs light, it is excited to a higher energy state. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different. Light Is Absorbed When Electrons.
From studiousguy.com
Examples of Objects That Absorb Light StudiousGuy Light Is Absorbed When Electrons Light absorption is the process in which light is absorbed and converted into energy. When an atom emits light, it decays to a lower energy state; When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. When an atom absorbs light, it is excited to a higher energy state. When an electron jumps from a.. Light Is Absorbed When Electrons.
From www.slideserve.com
PPT The Electron PowerPoint Presentation, free download ID6728644 Light Is Absorbed When Electrons Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c, of 2.998 × 10 8 m s −1. Light absorption is the process in which light is absorbed and converted into energy. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels.. Light Is Absorbed When Electrons.
From www.numerade.com
SOLVED determine the wavelength of the light absorbed when an electron Light Is Absorbed When Electrons When an atom absorbs light, it is excited to a higher energy state. Light absorption is the process in which light is absorbed and converted into energy. From fi reworks to stars, the color of light is useful in fi nding out what’s in matter. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are.. Light Is Absorbed When Electrons.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Light Is Absorbed When Electrons Light absorption is the process in which light is absorbed and converted into energy. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. When an electron jumps from a. When an atom emits light, it decays to a lower energy state; How does light reveal the behavior of electrons in an atom? When an. Light Is Absorbed When Electrons.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Light Is Absorbed When Electrons When an atom absorbs light, it is excited to a higher energy state. When an atom emits light, it decays to a lower energy state; When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c, of 2.998 × 10. Light Is Absorbed When Electrons.
From www.slideserve.com
PPT Electrons in Atoms PowerPoint Presentation, free download ID Light Is Absorbed When Electrons Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Light absorption is the process in which light is absorbed and converted into energy. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. When an atom absorbs light, it is excited to. Light Is Absorbed When Electrons.
From study.com
Electron Transition Definition, Chart & Examples Lesson Light Is Absorbed When Electrons When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c, of 2.998 × 10 8 m s −1. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy. Light Is Absorbed When Electrons.
From www.britannica.com
Light Emission, Absorption, Processes Britannica Light Is Absorbed When Electrons When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. When an atom emits light, it decays to a lower energy state; When an atom absorbs light, it is excited to a higher energy state. Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c, of 2.998 × 10. Light Is Absorbed When Electrons.
From www.slideserve.com
PPT Matter and Particles of Light Quantum Theory PowerPoint Light Is Absorbed When Electrons From fi reworks to stars, the color of light is useful in fi nding out what’s in matter. It's a common understanding that atoms emit or absorb light when the energy of the photons is equal to the the difference in the. When an atom absorbs light, it is excited to a higher energy state. When an electron jumps from. Light Is Absorbed When Electrons.
From www.youtube.com
What is the wavelength of light emitted when the electron in a hydrogen Light Is Absorbed When Electrons Light and other forms of electromagnetic radiation move through a vacuum with a constant speed, c, of 2.998 × 10 8 m s −1. From fi reworks to stars, the color of light is useful in fi nding out what’s in matter. It's a common understanding that atoms emit or absorb light when the energy of the photons is equal. Light Is Absorbed When Electrons.
From saylordotorg.github.io
Atomic Spectra and Models of the Atom Light Is Absorbed When Electrons How does light reveal the behavior of electrons in an atom? When an atom absorbs light, it is excited to a higher energy state. From fi reworks to stars, the color of light is useful in fi nding out what’s in matter. It's a common understanding that atoms emit or absorb light when the energy of the photons is equal. Light Is Absorbed When Electrons.
From webbtelescope.org
Spectroscopy 101 How Absorption and Emission Spectra Work b Light Is Absorbed When Electrons When an electron jumps from a. When an atom absorbs light, it is excited to a higher energy state. From fi reworks to stars, the color of light is useful in fi nding out what’s in matter. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. Light absorption is the process in which light. Light Is Absorbed When Electrons.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Light Is Absorbed When Electrons It's a common understanding that atoms emit or absorb light when the energy of the photons is equal to the the difference in the. From fi reworks to stars, the color of light is useful in fi nding out what’s in matter. When an atom absorbs light, it is excited to a higher energy state. When an atom emits light,. Light Is Absorbed When Electrons.
From www.slideserve.com
PPT Chapter 5 Light PowerPoint Presentation, free download ID2768132 Light Is Absorbed When Electrons From fi reworks to stars, the color of light is useful in fi nding out what’s in matter. When an atom emits light, it decays to a lower energy state; When an atom absorbs light, it is excited to a higher energy state. How does light reveal the behavior of electrons in an atom? Light and other forms of electromagnetic. Light Is Absorbed When Electrons.
From favpng.com
Light Fluorescence Electron Excitation Excited State, PNG, 793x779px Light Is Absorbed When Electrons When an electron jumps from a. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. When an atom absorbs light, it is excited to a higher energy state. When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are. How does light reveal. Light Is Absorbed When Electrons.
From www.slideserve.com
PPT Electrons and Quantum Theory PowerPoint Presentation, free Light Is Absorbed When Electrons When an atom absorbs light, it is excited to a higher energy state. When an electron jumps from a. When an atom emits light, it decays to a lower energy state; It's a common understanding that atoms emit or absorb light when the energy of the photons is equal to the the difference in the. Light absorption is the process. Light Is Absorbed When Electrons.