Why Does Xenon React With Fluorine Whereas Neon Does Not . But unlike neon, xenon has d orbitals that allow it to be surrounded by more than 8 electrons by. Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help accommodate electron. Xe has a lower ionization enthalpy than ne. Both xenon and neon, are in the full octate state. Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. Stable compounds of xenon form when xenon reacts with fluorine. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Its outer electrons then become a target for other. The only known binary compounds of noble gases are fluorides and oxides of xenon and to a lesse extent of kryton.
from www.numerade.com
Xe has a lower ionization enthalpy than ne. Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Its outer electrons then become a target for other. Stable compounds of xenon form when xenon reacts with fluorine. Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help accommodate electron. The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. Both xenon and neon, are in the full octate state. The only known binary compounds of noble gases are fluorides and oxides of xenon and to a lesse extent of kryton. Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly.
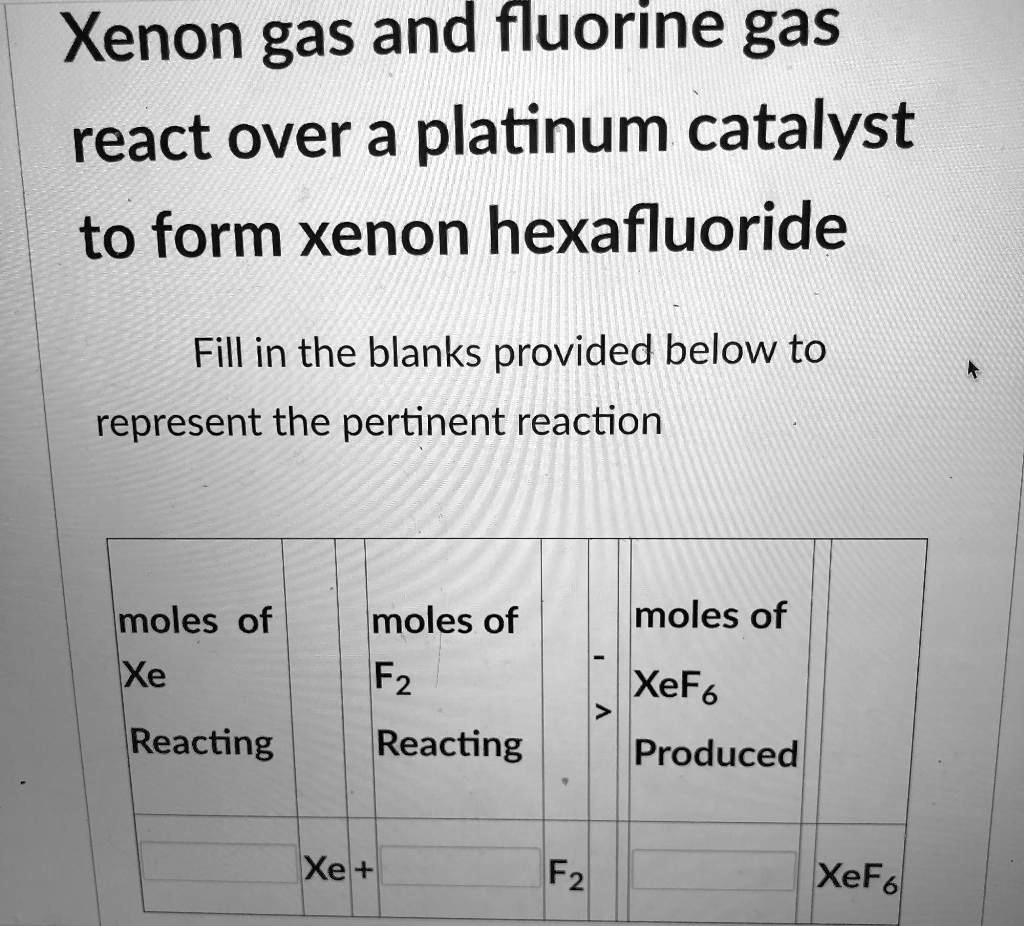
SOLVED Xenon gas and fluorine gas react over a platinum catalyst to
Why Does Xenon React With Fluorine Whereas Neon Does Not Xe has a lower ionization enthalpy than ne. Xe has a lower ionization enthalpy than ne. Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help accommodate electron. The only known binary compounds of noble gases are fluorides and oxides of xenon and to a lesse extent of kryton. Both xenon and neon, are in the full octate state. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. But unlike neon, xenon has d orbitals that allow it to be surrounded by more than 8 electrons by. The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Its outer electrons then become a target for other. Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. Stable compounds of xenon form when xenon reacts with fluorine.
From www.numerade.com
SOLVED (a) Why does xenon react with fluorine, whereas neon does not Why Does Xenon React With Fluorine Whereas Neon Does Not Stable compounds of xenon form when xenon reacts with fluorine. The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. But unlike neon, xenon has d orbitals that allow it to be surrounded by more than 8 electrons by. Xe has a lower ionization enthalpy than ne. Xenon forms compounds because its. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.numerade.com
SOLVEDDirect reaction of fluorine with xenon produces three different Why Does Xenon React With Fluorine Whereas Neon Does Not The only known binary compounds of noble gases are fluorides and oxides of xenon and to a lesse extent of kryton. Both xenon and neon, are in the full octate state. The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. Stable compounds of xenon form when xenon reacts with fluorine. Xenon. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID650195 Why Does Xenon React With Fluorine Whereas Neon Does Not Both xenon and neon, are in the full octate state. The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. But unlike neon, xenon has d orbitals that allow it to be surrounded by more than 8 electrons by.. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.numerade.com
Xenon and fluorine will react to form binary compounds when a mixture Why Does Xenon React With Fluorine Whereas Neon Does Not Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Both xenon and neon, are in the full octate state. Stable compounds of xenon form when xenon reacts with fluorine. Its outer electrons then become a target for other. The valence electrons in xe (n = 5) are much farther from the nucleus than those of. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.doubtnut.com
Why does fluorine not shown disproportionation reaction? Why Does Xenon React With Fluorine Whereas Neon Does Not Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help accommodate electron. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Its outer electrons. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.slideserve.com
PPT Group 18 Elements Noble Gases PowerPoint Presentation ID2168487 Why Does Xenon React With Fluorine Whereas Neon Does Not Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. But unlike neon, xenon has d orbitals that allow it to be surrounded by more than 8 electrons by. Xenon reacts with fluorine due to its larger atomic size and the greater shielding. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.numerade.com
SOLVED Xenon gas and fluorine gas react over a platinum catalyst to Why Does Xenon React With Fluorine Whereas Neon Does Not Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. But unlike neon, xenon has d orbitals that allow it to be surrounded by more than 8 electrons by. Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help accommodate electron. Its outer electrons then become a. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From periodictable.me
Fluorine Electron Configuration (F) with Orbital Diagram Why Does Xenon React With Fluorine Whereas Neon Does Not Stable compounds of xenon form when xenon reacts with fluorine. Both xenon and neon, are in the full octate state. Xe has a lower ionization enthalpy than ne. Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.numerade.com
⏩SOLVED(a) Why does xenon react with fluorine, whereas neon does Why Does Xenon React With Fluorine Whereas Neon Does Not The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help accommodate electron. Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. But unlike neon, xenon has d orbitals that. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.numerade.com
Consider the first ionization energy of neon and the electron affinity Why Does Xenon React With Fluorine Whereas Neon Does Not Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. But unlike neon, xenon has d orbitals that allow it to be surrounded by more than 8 electrons by. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Stable compounds of xenon. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.youtube.com
Why helium and neon do not form compounds with fluorine? 12 PBLOCK Why Does Xenon React With Fluorine Whereas Neon Does Not Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. Xe has a lower ionization enthalpy than ne. The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help accommodate electron.. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.slideserve.com
PPT CHEMISTRY 1000 PowerPoint Presentation, free download ID6083445 Why Does Xenon React With Fluorine Whereas Neon Does Not Xe has a lower ionization enthalpy than ne. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Stable compounds of xenon form when xenon reacts with fluorine. Its outer electrons then become a target for other. Xenon difluoride, xef 2, forms after heating an excess of. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.numerade.com
SOLVEDProvide an explanation for the observation that helium, neon Why Does Xenon React With Fluorine Whereas Neon Does Not But unlike neon, xenon has d orbitals that allow it to be surrounded by more than 8 electrons by. Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. Both xenon and neon, are in the full octate state. Its outer electrons then become a target for other. Xenon forms compounds because its inner electrons screen the. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID650195 Why Does Xenon React With Fluorine Whereas Neon Does Not Its outer electrons then become a target for other. Both xenon and neon, are in the full octate state. Stable compounds of xenon form when xenon reacts with fluorine. The only known binary compounds of noble gases are fluorides and oxides of xenon and to a lesse extent of kryton. The valence electrons in xe (n = 5) are much. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From valenceelectrons.com
Fluorine Electron Configuration With Full Orbital Diagram Why Does Xenon React With Fluorine Whereas Neon Does Not Stable compounds of xenon form when xenon reacts with fluorine. The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. But unlike neon, xenon has d orbitals that allow it to be surrounded by more than 8 electrons by. Its outer electrons then become a target for other. Xenon forms compounds because. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.numerade.com
SOLVEDWhy does xenon react with fluorine, whereas neon does not? Why Does Xenon React With Fluorine Whereas Neon Does Not Stable compounds of xenon form when xenon reacts with fluorine. The only known binary compounds of noble gases are fluorides and oxides of xenon and to a lesse extent of kryton. Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help accommodate electron. The valence electrons in xe (n = 5). Why Does Xenon React With Fluorine Whereas Neon Does Not.
From periodictable.me
Xenon Electron Configuration Archives Dynamic Periodic Table of Why Does Xenon React With Fluorine Whereas Neon Does Not The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Stable compounds of xenon form when xenon reacts with fluorine. Both xenon and neon, are in the full octate. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From pediaa.com
Difference Between Fluorine and Fluoride Definition, Properties Why Does Xenon React With Fluorine Whereas Neon Does Not Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Xe has a lower ionization enthalpy than ne. Its outer electrons then become a target for other. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. The only known binary compounds of. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.numerade.com
SOLVEDThe reaction of three molecules of fluorine gas with a Xe atom Why Does Xenon React With Fluorine Whereas Neon Does Not Its outer electrons then become a target for other. Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help accommodate electron. Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Both xenon and neon, are in the full octate state. But unlike neon, xenon has d. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From slideplayer.com
WHY DO ATOMS REACT?. ppt download Why Does Xenon React With Fluorine Whereas Neon Does Not The only known binary compounds of noble gases are fluorides and oxides of xenon and to a lesse extent of kryton. Its outer electrons then become a target for other. Both xenon and neon, are in the full octate state. Stable compounds of xenon form when xenon reacts with fluorine. Xenon forms compounds because its inner electrons screen the outer. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.numerade.com
SOLVED(a) Why does xenon react with fluorine, whereas neon does not Why Does Xenon React With Fluorine Whereas Neon Does Not The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. Stable compounds of xenon form when xenon reacts with fluorine. Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. Xe has a lower ionization. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.numerade.com
The illustration to the left represents a mixture of xenon brown and Why Does Xenon React With Fluorine Whereas Neon Does Not The only known binary compounds of noble gases are fluorides and oxides of xenon and to a lesse extent of kryton. Both xenon and neon, are in the full octate state. Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From material-properties.org
Fluorine and Neon Comparison Properties Material Properties Why Does Xenon React With Fluorine Whereas Neon Does Not The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. Stable compounds of xenon form when xenon reacts with fluorine. Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. The only known binary compounds. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.nuclear-power.com
Fluorine Electron Affinity Electronegativity Ionization Energy of Why Does Xenon React With Fluorine Whereas Neon Does Not Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Stable compounds of xenon form when xenon reacts with fluorine. Xe has a lower ionization enthalpy than ne. Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. Xenon forms compounds because its inner. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.youtube.com
Xenon fluorine compounds YouTube Why Does Xenon React With Fluorine Whereas Neon Does Not Its outer electrons then become a target for other. Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. The only known binary compounds of noble gases are fluorides and oxides of xenon and. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.numerade.com
Why does xenon form stable compounds with fluorine, whereas argon does Why Does Xenon React With Fluorine Whereas Neon Does Not Xe has a lower ionization enthalpy than ne. Stable compounds of xenon form when xenon reacts with fluorine. Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help accommodate electron. The only known binary compounds of noble. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.slideserve.com
PPT CHEMISTRY 1000 PowerPoint Presentation, free download ID6083445 Why Does Xenon React With Fluorine Whereas Neon Does Not Stable compounds of xenon form when xenon reacts with fluorine. Both xenon and neon, are in the full octate state. Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help accommodate electron. The only known binary compounds of noble gases are fluorides and oxides of xenon and to a lesse extent. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.doubtnut.com
[Odia] Why does xenon form compounds only with fluorine and oxygen Why Does Xenon React With Fluorine Whereas Neon Does Not Both xenon and neon, are in the full octate state. Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Stable compounds of xenon form when xenon reacts with fluorine. Xe has a lower ionization enthalpy than ne. The only known binary compounds of noble gases are fluorides and oxides of xenon and to a lesse. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From studiousguy.com
Xenon (Xe) Properties & Uses StudiousGuy Why Does Xenon React With Fluorine Whereas Neon Does Not Stable compounds of xenon form when xenon reacts with fluorine. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Xe has a lower ionization enthalpy than ne. Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.numerade.com
SOLVED Although xenon is a “noble gas,” it reacts with fluorine and Why Does Xenon React With Fluorine Whereas Neon Does Not Stable compounds of xenon form when xenon reacts with fluorine. Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.numerade.com
SOLVED (a) Why does xenon react with fluorine, whereas neon does not Why Does Xenon React With Fluorine Whereas Neon Does Not Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help accommodate electron. The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. The only known binary compounds of noble gases are fluorides and oxides of xenon and to a lesse extent of kryton.. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.animalia-life.club
Neon Orbital Notation Why Does Xenon React With Fluorine Whereas Neon Does Not Both xenon and neon, are in the full octate state. Its outer electrons then become a target for other. Xenon difluoride, xef 2, forms after heating an excess of xenon gas with. Furthermore, xenon (xe) has lower ionization energy leading to a chemical bond and has empty orbitals that can help accommodate electron. Xe has a lower ionization enthalpy than. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From slideplayer.com
Elemental Properties and Patterns ppt download Why Does Xenon React With Fluorine Whereas Neon Does Not Xe has a lower ionization enthalpy than ne. The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From www.labroots.com
Reactions Impossible How Chemists Strived to Make Noble Gas Compounds Why Does Xenon React With Fluorine Whereas Neon Does Not Both xenon and neon, are in the full octate state. But unlike neon, xenon has d orbitals that allow it to be surrounded by more than 8 electrons by. The only known binary compounds of noble gases are fluorides and oxides of xenon and to a lesse extent of kryton. Xenon difluoride, xef 2, forms after heating an excess of. Why Does Xenon React With Fluorine Whereas Neon Does Not.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Why Does Xenon React With Fluorine Whereas Neon Does Not Stable compounds of xenon form when xenon reacts with fluorine. Xe has a lower ionization enthalpy than ne. The only known binary compounds of noble gases are fluorides and oxides of xenon and to a lesse extent of kryton. The valence electrons in xe (n = 5) are much farther from the nucleus than those of ne. Both xenon and. Why Does Xenon React With Fluorine Whereas Neon Does Not.