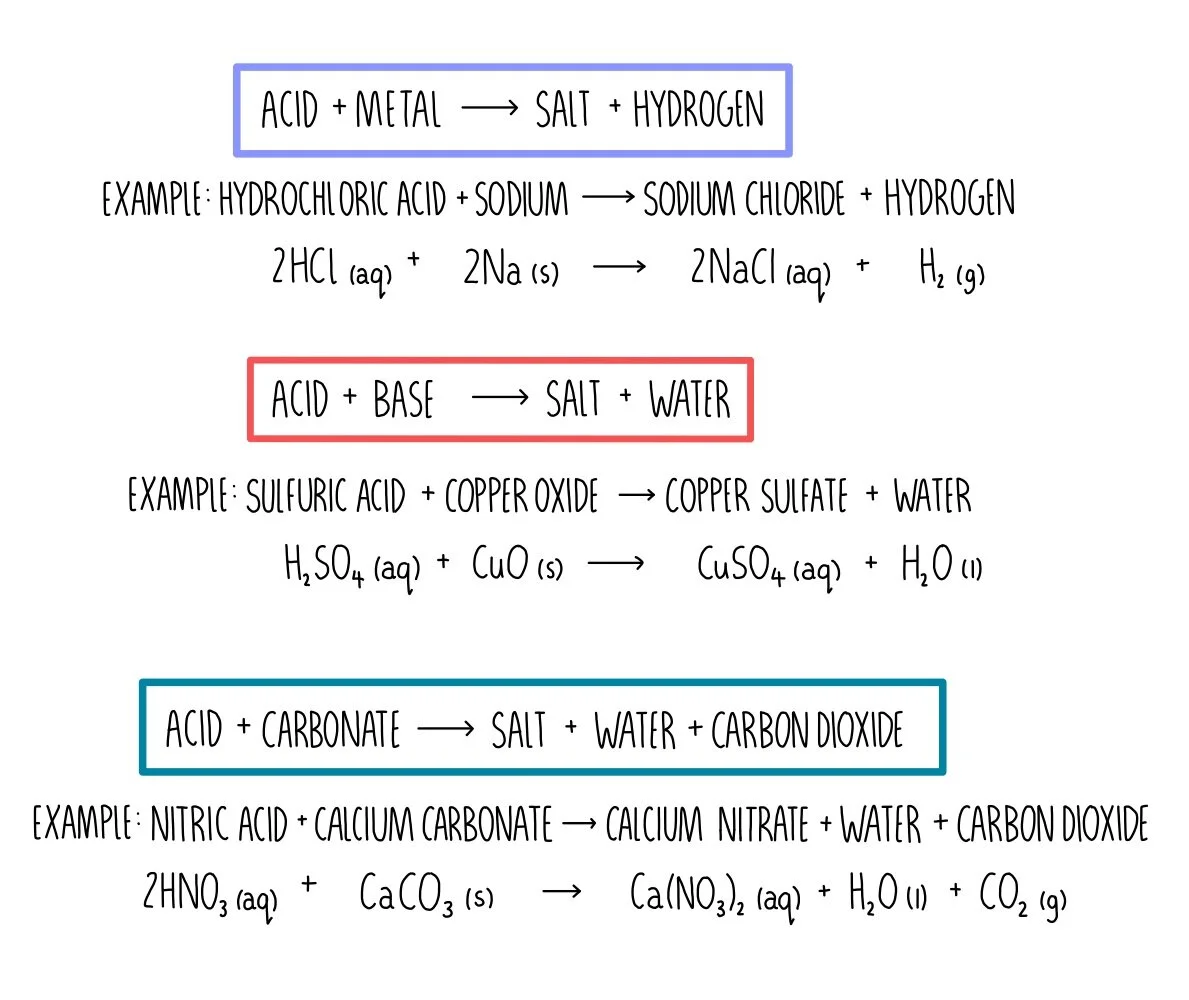
Is Ammonium Base Or Acid . Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Also, when dissolved in water,. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. The reactions of amines with acids.
from www.thesciencehive.co.uk
Also, when dissolved in water,. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. The reactions of amines with acids. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker.
Acids, Bases and Salt Preparations (GCSE) — the science hive
Is Ammonium Base Or Acid The reactions of amines with acids. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Also, when dissolved in water,. The reactions of amines with acids.
From www.scienceabc.com
Is Ammonia An Acid Or Base? » ScienceABC Is Ammonium Base Or Acid Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. The reactions of amines with acids. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Ammonia is a weak base because its nitrogen atom. Is Ammonium Base Or Acid.
From www.slideserve.com
PPT Chapter 19 Acids, Bases, and Salts PowerPoint Presentation, free Is Ammonium Base Or Acid Also, when dissolved in water,. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. The reactions of amines with acids. Ammonia is a weak. Is Ammonium Base Or Acid.
From www.slideserve.com
PPT IX. Acids, Bases and Salts PowerPoint Presentation, free download Is Ammonium Base Or Acid Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. The reactions of amines with acids. Also, when dissolved in water,. Acidic salt (strong acid/weak base) these are acidic. Is Ammonium Base Or Acid.
From byjus.com
nh4cl is acid but any NH2 is base in liquid ammonia Is Ammonium Base Or Acid Also, when dissolved in water,. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. The reactions of amines with acids. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Ammonia is a weak. Is Ammonium Base Or Acid.
From jonathanareschapman.blogspot.com
Ammonia Acid or Base JonathanaresChapman Is Ammonium Base Or Acid Also, when dissolved in water,. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. The reactions of amines with acids. Similarly, in the reaction of ammonia with water, the. Is Ammonium Base Or Acid.
From www.chemicals.co.uk
The Surprising Reaction Of Nitric Acid & Ammonia The Chemistry Blog Is Ammonium Base Or Acid Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Also, when dissolved in water,. Ammonia is a weak base because its nitrogen atom has. Is Ammonium Base Or Acid.
From www.youtube.com
Equation for NH3 + H2O (Ammonia + Water) YouTube Is Ammonium Base Or Acid Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Also, when dissolved in water,. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate. Is Ammonium Base Or Acid.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Is Ammonium Base Or Acid Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Also, when dissolved in water,. The reactions of amines with acids. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Acidic salt (strong acid/weak base) these are acidic. Is Ammonium Base Or Acid.
From solutionpharmacy.in
Assay of Ammonium Chloride by Acid Base Titration Solution Parmacy Is Ammonium Base Or Acid Also, when dissolved in water,. The reactions of amines with acids. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Ammonia is a weak. Is Ammonium Base Or Acid.
From sank-ka.blogspot.com
Ammonia Acid Or Base Acids and Bases ADVoscience Many substances Is Ammonium Base Or Acid Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Also, when dissolved in water,. The reactions of amines with acids. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Similarly, in the reaction of ammonia with water, the. Is Ammonium Base Or Acid.
From techiescientist.com
Is NH3 (Ammonia) an Acid or a Base? Techiescientist Is Ammonium Base Or Acid Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. The reactions of amines with acids. Ammonia is a weak base because its nitrogen atom. Is Ammonium Base Or Acid.
From techiescientist.com
Is NH4+ an Acid or Base? Techiescientist Is Ammonium Base Or Acid Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Also, when dissolved in water,. The reactions of amines with acids. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Acidic salt (strong acid/weak base) these are acidic. Is Ammonium Base Or Acid.
From techiescientist.com
Is NH3 (Ammonia) an Acid or a Base? Techiescientist Is Ammonium Base Or Acid Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Also, when dissolved in water,. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. The reactions of amines with acids. Ammonia is a weak. Is Ammonium Base Or Acid.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID Is Ammonium Base Or Acid Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Also, when dissolved in water,. The reactions of amines with acids. Acidic salt (strong acid/weak base) these are acidic. Is Ammonium Base Or Acid.
From www.alamy.com
Ammonium compounds ammonium bicarbonate, ammonium carbamate, ammonium Is Ammonium Base Or Acid Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base,. Is Ammonium Base Or Acid.
From pisifqdmjp.blogspot.com
Ammonium Chloride Formula Acid And Base IB Biology/Chemistry IB Is Ammonium Base Or Acid Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Ammonia is a weak base because its nitrogen atom has an electron pair that readily. Is Ammonium Base Or Acid.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Is Ammonium Base Or Acid Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. The reactions of amines with acids. Acidic salt (strong acid/weak base) these are acidic since the cation is the. Is Ammonium Base Or Acid.
From beingteaching.com
The Stunning Response Of Nitric Acid & Ammonia Being Teaching 2024 Is Ammonium Base Or Acid Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Also, when dissolved in water,. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate. Is Ammonium Base Or Acid.
From courses.lumenlearning.com
Buffers Chemistry Atoms First Is Ammonium Base Or Acid Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Also, when dissolved in water,. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base,. Is Ammonium Base Or Acid.
From www.youtube.com
Ammonium Chloride Acid Base Reaction chemistry chemicalreaction Is Ammonium Base Or Acid Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. The reactions of amines with acids. Also, when dissolved in water,. Similarly, in the reaction of ammonia with water, the. Is Ammonium Base Or Acid.
From h-o-m-e.org
Ammonium Chloride Is it Acidic or Basic? Is Ammonium Base Or Acid Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Ammonia is a weak base because its nitrogen atom has an electron pair that readily. Is Ammonium Base Or Acid.
From www.scienceabc.com
Is Ammonia An Acid Or Base? » ScienceABC Is Ammonium Base Or Acid Also, when dissolved in water,. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Ammonia is a weak base because its nitrogen atom has. Is Ammonium Base Or Acid.
From topblogtenz.com
Is NH4 an acid or base? Strong vs Weak Ammonium ion Is Ammonium Base Or Acid Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Also, when dissolved in water,. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base,. Is Ammonium Base Or Acid.
From www.visionlearning.com
Acids and Bases II Chemistry Visionlearning Is Ammonium Base Or Acid Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. The reactions of amines with acids. Also, when dissolved in water,. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Similarly, in the reaction of ammonia with water, the. Is Ammonium Base Or Acid.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2089759 Is Ammonium Base Or Acid Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Also, when dissolved in water,. The reactions of amines with acids. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Similarly, in the reaction of ammonia with water, the. Is Ammonium Base Or Acid.
From www.slideserve.com
PPT To understand two models of acids and bases PowerPoint Is Ammonium Base Or Acid Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. The reactions of amines with acids. Also, when dissolved in water,. Similarly, in the reaction of ammonia with water, the. Is Ammonium Base Or Acid.
From yadielsen.blogspot.com
Nh3 Acid Or Base Ammonia is highly soluble in water while nitrogen is Is Ammonium Base Or Acid Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base,. Is Ammonium Base Or Acid.
From www.shutterstock.com
Acid Base Chemistry Solutions Ammonium Hydroxide Stock Photo (Edit Now Is Ammonium Base Or Acid Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak. Is Ammonium Base Or Acid.
From sciencenotes.org
The pH Scale of Common Chemicals Is Ammonium Base Or Acid Also, when dissolved in water,. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. The reactions of amines with acids. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Acidic salt (strong acid/weak base) these are acidic. Is Ammonium Base Or Acid.
From www.slideserve.com
PPT NON AQUEOUS SOLVENTS PowerPoint Presentation, free download ID Is Ammonium Base Or Acid The reactions of amines with acids. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Also, when dissolved in water,. Ammonia is a weak. Is Ammonium Base Or Acid.
From www.youtube.com
Is NH3 (Ammonia) an Acid, Base, or Neutral? YouTube Is Ammonium Base Or Acid Also, when dissolved in water,. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate. Is Ammonium Base Or Acid.
From sank-ka.blogspot.com
Ammonia Acid Or Base Acids and Bases ADVoscience Many substances Is Ammonium Base Or Acid Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Also, when dissolved in water,. The reactions of amines with acids. Ammonia is a weak. Is Ammonium Base Or Acid.
From www.studypool.com
SOLUTION Assay of ammonium chloride by acid base titration 1 Studypool Is Ammonium Base Or Acid Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Also, when dissolved in water,. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate. Is Ammonium Base Or Acid.
From discover.hubpages.com
The Difference in the Chemistry of Hydrolysis & Hydration. HubPages Is Ammonium Base Or Acid Also, when dissolved in water,. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. The reactions of amines with acids. Acidic salt (strong acid/weak base) these are acidic. Is Ammonium Base Or Acid.
From www.youtube.com
Conjugate Acid of NH3 YouTube Is Ammonium Base Or Acid Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the. The reactions of amines with acids. Also, when dissolved in water,. Ammonia is a weak. Is Ammonium Base Or Acid.