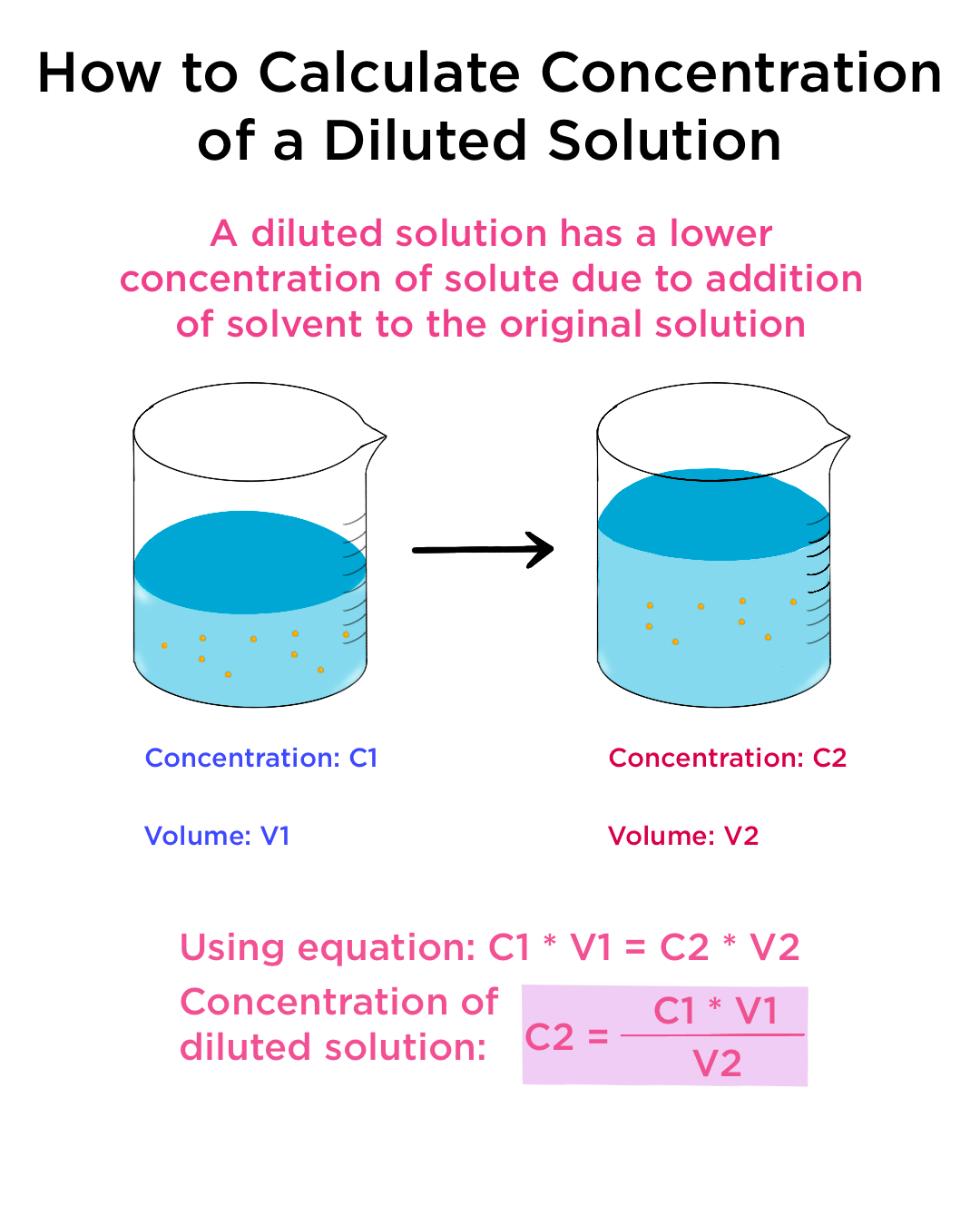
Dilute Vs Concentrated . Dilution is a process used to lower the concentration of the original solution by adding more solvent. The major component of a solution is called the solvent, and the minor. A concentrated solution is one that has a relatively large amount of dissolved solute. A dilute solution has less solute than a concentrated solution, which has more solute. Learn how to dilute a solution by adding solvent to it, and how to calculate the concentration of the diluted solution. Learn the difference between dilute and concentrated solutions, two types of homogeneous mixtures. A dilute solution is one that has a relatively small. Learn the difference between dilute and concentrated solutions with examples and definitions. What is the difference between a dilute and concentrated solution? A solution is a homogeneous mixture of two or more substances. A dilute solution has a low solute concentration (i.e., a concentration significantly. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. A dilute solution has low solute.
from ar.inspiredpencil.com
A concentrated solution is one that has a relatively large amount of dissolved solute. What is the difference between a dilute and concentrated solution? A dilute solution has a low solute concentration (i.e., a concentration significantly. A dilute solution has low solute. A solution is a homogeneous mixture of two or more substances. Learn the difference between dilute and concentrated solutions with examples and definitions. Learn how to dilute a solution by adding solvent to it, and how to calculate the concentration of the diluted solution. Concentration is the removal of solvent, which increases the concentration of. A dilute solution has less solute than a concentrated solution, which has more solute. A dilute solution is one that has a relatively small.
Concentrated Vs Dilute Solutions
Dilute Vs Concentrated A dilute solution has low solute. The major component of a solution is called the solvent, and the minor. A dilute solution has less solute than a concentrated solution, which has more solute. Learn how to dilute a solution by adding solvent to it, and how to calculate the concentration of the diluted solution. Learn the difference between dilute and concentrated solutions, two types of homogeneous mixtures. Learn the difference between dilute and concentrated solutions with examples and definitions. A dilute solution has low solute. What is the difference between a dilute and concentrated solution? Concentration is the removal of solvent, which increases the concentration of. A dilute solution is one that has a relatively small. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution has a low solute concentration (i.e., a concentration significantly. Dilution is a process used to lower the concentration of the original solution by adding more solvent. A solution is a homogeneous mixture of two or more substances. A concentrated solution is one that has a relatively large amount of dissolved solute.
From www.slideserve.com
PPT Solution Concentration PowerPoint Presentation, free download Dilute Vs Concentrated A dilute solution has a low solute concentration (i.e., a concentration significantly. A dilute solution is one that has a relatively small. Learn the difference between dilute and concentrated solutions, two types of homogeneous mixtures. Dilution is a process used to lower the concentration of the original solution by adding more solvent. The major component of a solution is called. Dilute Vs Concentrated.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Dilute Vs Concentrated Learn the difference between dilute and concentrated solutions with examples and definitions. A dilute solution has less solute than a concentrated solution, which has more solute. A solution is a homogeneous mixture of two or more substances. Learn the difference between dilute and concentrated solutions, two types of homogeneous mixtures. Dilution is a process used to lower the concentration of. Dilute Vs Concentrated.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Vs Concentrated Dilution is a process used to lower the concentration of the original solution by adding more solvent. A dilute solution has a low solute concentration (i.e., a concentration significantly. What is the difference between a dilute and concentrated solution? Learn the difference between dilute and concentrated solutions, two types of homogeneous mixtures. Dilution is the addition of solvent, which decreases. Dilute Vs Concentrated.
From www.showme.com
Concentrated vs. Diluted Solution Science ShowMe Dilute Vs Concentrated Concentration is the removal of solvent, which increases the concentration of. A solution is a homogeneous mixture of two or more substances. Learn the difference between dilute and concentrated solutions with examples and definitions. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Dilution is the addition of solvent, which decreases the. Dilute Vs Concentrated.
From www.youtube.com
Chemical Solutions Dilute vs. Concentrated YouTube Dilute Vs Concentrated Learn the difference between dilute and concentrated solutions, two types of homogeneous mixtures. Learn how to dilute a solution by adding solvent to it, and how to calculate the concentration of the diluted solution. A dilute solution has low solute. What is the difference between a dilute and concentrated solution? Dilution is a process used to lower the concentration of. Dilute Vs Concentrated.
From www.slideserve.com
PPT MEMBRANE PERMEABILITY PowerPoint Presentation, free download ID Dilute Vs Concentrated A dilute solution has less solute than a concentrated solution, which has more solute. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to dilute a solution by adding solvent to it, and how to calculate the concentration of the diluted solution. Learn the difference between dilute and concentrated solutions,. Dilute Vs Concentrated.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Dilute Vs Concentrated What is the difference between a dilute and concentrated solution? Dilution is a process used to lower the concentration of the original solution by adding more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one that has a relatively small. The major component of a solution is. Dilute Vs Concentrated.
From www.slideserve.com
PPT Investigating Solutions PowerPoint Presentation, free download Dilute Vs Concentrated The major component of a solution is called the solvent, and the minor. Learn the difference between dilute and concentrated solutions with examples and definitions. Concentration is the removal of solvent, which increases the concentration of. What is the difference between a dilute and concentrated solution? Dilution is the addition of solvent, which decreases the concentration of the solute in. Dilute Vs Concentrated.
From www.slideserve.com
PPT Concentration …a measure of solutetosolvent ratio concentrated Dilute Vs Concentrated A solution is a homogeneous mixture of two or more substances. A dilute solution is one that has a relatively small. Learn the difference between dilute and concentrated solutions, two types of homogeneous mixtures. What is the difference between a dilute and concentrated solution? Learn the difference between dilute and concentrated solutions with examples and definitions. Learn how to dilute. Dilute Vs Concentrated.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Vs Concentrated Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution has low solute. A dilute solution is one that has a relatively small. The major component of a solution is called the solvent, and the minor. A dilute solution has less solute than a concentrated solution, which has more solute. Concentration. Dilute Vs Concentrated.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Vs Concentrated A dilute solution is one that has a relatively small. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution has low solute. A dilute solution has less solute than a concentrated solution, which. Dilute Vs Concentrated.
From brainly.ph
What is the difference between a dilute solution and a concentrated Dilute Vs Concentrated Concentration is the removal of solvent, which increases the concentration of. What is the difference between a dilute and concentrated solution? A dilute solution has less solute than a concentrated solution, which has more solute. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution has a low solute concentration (i.e.,. Dilute Vs Concentrated.
From www.youtube.com
Solutions concentrated vs. dilute YouTube Dilute Vs Concentrated A solution is a homogeneous mixture of two or more substances. A concentrated solution is one that has a relatively large amount of dissolved solute. The major component of a solution is called the solvent, and the minor. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. What is the difference between a. Dilute Vs Concentrated.
From www.youtube.com
Dilute and Concentrated Solution YouTube Dilute Vs Concentrated A concentrated solution is one that has a relatively large amount of dissolved solute. A dilute solution has a low solute concentration (i.e., a concentration significantly. A dilute solution has less solute than a concentrated solution, which has more solute. A dilute solution has low solute. Dilution is a process used to lower the concentration of the original solution by. Dilute Vs Concentrated.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Dilute Vs Concentrated The major component of a solution is called the solvent, and the minor. What is the difference between a dilute and concentrated solution? A dilute solution has less solute than a concentrated solution, which has more solute. A dilute solution has a low solute concentration (i.e., a concentration significantly. Learn how to dilute a solution by adding solvent to it,. Dilute Vs Concentrated.
From www.youtube.com
Dilute vs. Concentrated YouTube Dilute Vs Concentrated A dilute solution is one that has a relatively small. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. What is the difference between a dilute and concentrated solution? A dilute solution has low solute. A solution is a homogeneous mixture of two or more substances. Concentration is the removal of solvent, which. Dilute Vs Concentrated.
From ycscience9.weebly.com
Concentrated vs. Diluted Y9 Science Dilute Vs Concentrated Learn how to dilute a solution by adding solvent to it, and how to calculate the concentration of the diluted solution. A concentrated solution is one that has a relatively large amount of dissolved solute. A dilute solution has less solute than a concentrated solution, which has more solute. What is the difference between a dilute and concentrated solution? Dilution. Dilute Vs Concentrated.
From slideplayer.com
Solution Concentration ppt download Dilute Vs Concentrated A dilute solution is one that has a relatively small. A dilute solution has low solute. A solution is a homogeneous mixture of two or more substances. Dilution is a process used to lower the concentration of the original solution by adding more solvent. A dilute solution has a low solute concentration (i.e., a concentration significantly. Dilution is the addition. Dilute Vs Concentrated.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Vs Concentrated A dilute solution has a low solute concentration (i.e., a concentration significantly. Learn the difference between dilute and concentrated solutions with examples and definitions. A concentrated solution is one that has a relatively large amount of dissolved solute. The major component of a solution is called the solvent, and the minor. Dilution is a process used to lower the concentration. Dilute Vs Concentrated.
From www.istockphoto.com
Concentrated Solution Vs Dilute Solution Stock Illustration Download Dilute Vs Concentrated A concentrated solution is one that has a relatively large amount of dissolved solute. What is the difference between a dilute and concentrated solution? Learn the difference between dilute and concentrated solutions with examples and definitions. Learn how to dilute a solution by adding solvent to it, and how to calculate the concentration of the diluted solution. A solution is. Dilute Vs Concentrated.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID4509517 Dilute Vs Concentrated A concentrated solution is one that has a relatively large amount of dissolved solute. The major component of a solution is called the solvent, and the minor. What is the difference between a dilute and concentrated solution? A dilute solution has low solute. Learn the difference between dilute and concentrated solutions with examples and definitions. Learn the difference between dilute. Dilute Vs Concentrated.
From slideplayer.com
Solutions Unit. ppt download Dilute Vs Concentrated What is the difference between a dilute and concentrated solution? A dilute solution has a low solute concentration (i.e., a concentration significantly. A dilute solution has low solute. The major component of a solution is called the solvent, and the minor. Learn the difference between dilute and concentrated solutions with examples and definitions. A dilute solution has less solute than. Dilute Vs Concentrated.
From slideplayer.com
Solutions Unit. ppt download Dilute Vs Concentrated Concentration is the removal of solvent, which increases the concentration of. Learn how to dilute a solution by adding solvent to it, and how to calculate the concentration of the diluted solution. Learn the difference between dilute and concentrated solutions with examples and definitions. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution.. Dilute Vs Concentrated.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Vs Concentrated A dilute solution has a low solute concentration (i.e., a concentration significantly. What is the difference between a dilute and concentrated solution? Learn how to dilute a solution by adding solvent to it, and how to calculate the concentration of the diluted solution. A dilute solution has low solute. A solution is a homogeneous mixture of two or more substances.. Dilute Vs Concentrated.
From www.slideserve.com
PPT Dilute and Concentrated Solutions PowerPoint Presentation, free Dilute Vs Concentrated A concentrated solution is one that has a relatively large amount of dissolved solute. A dilute solution has low solute. The major component of a solution is called the solvent, and the minor. What is the difference between a dilute and concentrated solution? A solution is a homogeneous mixture of two or more substances. A dilute solution has a low. Dilute Vs Concentrated.
From slideplayer.com
Solution Concentration ppt download Dilute Vs Concentrated A concentrated solution is one that has a relatively large amount of dissolved solute. Learn the difference between dilute and concentrated solutions with examples and definitions. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The major component of a solution is called the solvent, and the minor. Learn how to dilute a. Dilute Vs Concentrated.
From www.slideserve.com
PPT Concentration Calculations PowerPoint Presentation, free download Dilute Vs Concentrated Learn the difference between dilute and concentrated solutions with examples and definitions. Learn the difference between dilute and concentrated solutions, two types of homogeneous mixtures. A dilute solution has less solute than a concentrated solution, which has more solute. What is the difference between a dilute and concentrated solution? Dilution is a process used to lower the concentration of the. Dilute Vs Concentrated.
From www.slideserve.com
PPT Chapter 15 Solutions PowerPoint Presentation, free download ID Dilute Vs Concentrated A dilute solution is one that has a relatively small. Learn the difference between dilute and concentrated solutions, two types of homogeneous mixtures. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A solution is a homogeneous mixture of two or more. Dilute Vs Concentrated.
From www.geeksforgeeks.org
Concentration of Solution Definition, Formulas & Solved Examples Dilute Vs Concentrated Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. What is the difference between a dilute and concentrated solution? Learn the difference between dilute and concentrated solutions with examples and definitions. Learn how to dilute a solution by adding solvent to it,. Dilute Vs Concentrated.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Vs Concentrated Learn the difference between dilute and concentrated solutions, two types of homogeneous mixtures. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute a solution by adding solvent to it, and how to calculate the concentration of the diluted solution. A dilute solution has less solute than a concentrated solution,. Dilute Vs Concentrated.
From www.onlinenotesnepal.com
Mixture, Solubility, Dilute and concentrated solution in Grade 9 Dilute Vs Concentrated Learn the difference between dilute and concentrated solutions, two types of homogeneous mixtures. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute a solution by adding solvent to it, and how to calculate the concentration of the diluted solution. Dilution is a process used to lower the concentration of. Dilute Vs Concentrated.
From www.slideserve.com
PPT Measuring Concentration PowerPoint Presentation, free download Dilute Vs Concentrated Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn the difference between dilute and concentrated solutions with examples and definitions. A dilute solution has low solute. Learn the difference between dilute and concentrated solutions, two types of homogeneous mixtures. A concentrated solution is one that has a relatively large amount of dissolved. Dilute Vs Concentrated.
From slideplayer.com
Concentration of a Solution ppt download Dilute Vs Concentrated Learn how to dilute a solution by adding solvent to it, and how to calculate the concentration of the diluted solution. A solution is a homogeneous mixture of two or more substances. The major component of a solution is called the solvent, and the minor. What is the difference between a dilute and concentrated solution? A dilute solution has a. Dilute Vs Concentrated.
From www.askdifference.com
Dilute Solution vs. Concentrated Solution — What’s the Difference? Dilute Vs Concentrated What is the difference between a dilute and concentrated solution? A dilute solution has low solute. Dilution is a process used to lower the concentration of the original solution by adding more solvent. A solution is a homogeneous mixture of two or more substances. A dilute solution has less solute than a concentrated solution, which has more solute. Learn how. Dilute Vs Concentrated.
From www.slideserve.com
PPT Solutions Part II PowerPoint Presentation, free download ID4272422 Dilute Vs Concentrated A concentrated solution is one that has a relatively large amount of dissolved solute. Dilution is a process used to lower the concentration of the original solution by adding more solvent. A dilute solution is one that has a relatively small. A dilute solution has low solute. What is the difference between a dilute and concentrated solution? Concentration is the. Dilute Vs Concentrated.