Medical Device Development Tools Fda . The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing innovative medical device. Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. Food and drug administration (fda) to. Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. The medical device development tool (mddt) program is a way for the u.s. The medical device development tools (mddt) program is a formal cdrh program that encourages stakeholders to propose and. The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. Ai & machine learningdedicated dev teams
from www.iqvia.com
The medical device development tool (mddt) program is a way for the u.s. Food and drug administration (fda) to. The medical device development tools (mddt) program is a formal cdrh program that encourages stakeholders to propose and. The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing innovative medical device. Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. Ai & machine learningdedicated dev teams The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical.
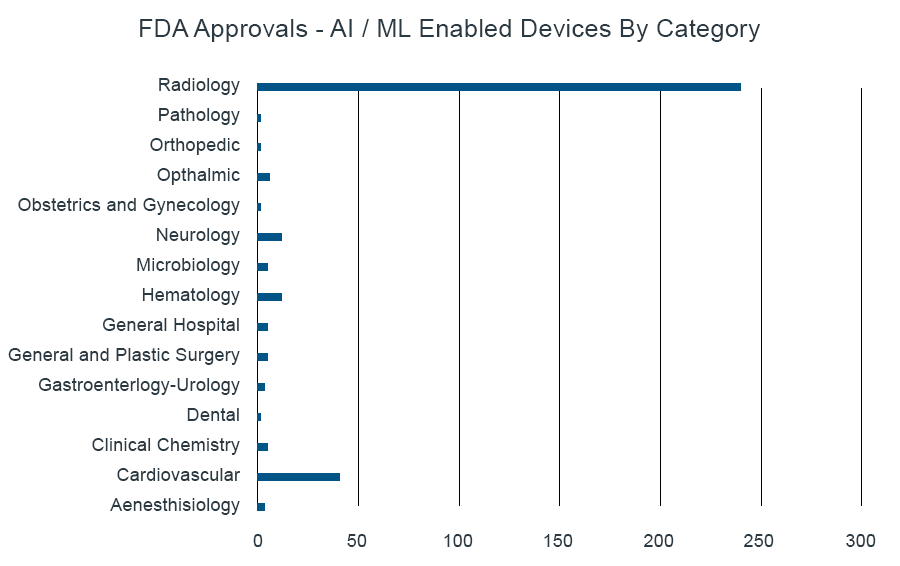
FDA Publishes Approved List of AI/MLenabled Medical Devices IQVIA
Medical Device Development Tools Fda Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. The medical device development tool (mddt) program is a way for the u.s. Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. Ai & machine learningdedicated dev teams Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing innovative medical device. Food and drug administration (fda) to. The medical device development tools (mddt) program is a formal cdrh program that encourages stakeholders to propose and.
From emmainternational.com
FDA’s Catalog of Regulatory Science Tools EMMA International Medical Device Development Tools Fda The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. The us. Medical Device Development Tools Fda.
From webinars.sw.siemens.com
Navigating the FDA Approval Process for Your Software Based Medical De Medical Device Development Tools Fda The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. The medical device development tools (mddt) program is a formal cdrh program that encourages stakeholders to propose and. The fda center for devices and radiological. Medical Device Development Tools Fda.
From www.slideserve.com
PPT Medical Device Development Tools FDA CDRH Pilot Program Medical Device Development Tools Fda The medical device development tools (mddt) program is a formal cdrh program that encourages stakeholders to propose and. Ai & machine learningdedicated dev teams The medical device development tool (mddt) program is a way for the u.s. The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. The us food and drug. Medical Device Development Tools Fda.
From www.vrogue.co
Understanding The 7 Phases Of Medical Device Developm vrogue.co Medical Device Development Tools Fda Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. Ai & machine learningdedicated dev teams The medical device development tool (mddt) program is a way for the u.s. The medical device development tools. Medical Device Development Tools Fda.
From www.vrogue.co
Understanding The 7 Phases Of Medical Device Developm vrogue.co Medical Device Development Tools Fda The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. The medical device development tools (mddt) program is a formal cdrh program that encourages stakeholders to propose and. Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. Using this program,. Medical Device Development Tools Fda.
From www.slideteam.net
Medical Device Development And Commercialization Process PPT PowerPoint Medical Device Development Tools Fda Food and drug administration (fda) to. Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. Ai. Medical Device Development Tools Fda.
From www.biostatistik-consulting.ch
FDA's Medical Device Development Tools Program Biostatistik Schweiz Medical Device Development Tools Fda Ai & machine learningdedicated dev teams The medical device development tools (mddt) program is a formal cdrh program that encourages stakeholders to propose and. Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. The program aims to provide mddt developers and medical device sponsors with a mechanism. Medical Device Development Tools Fda.
From www.slideserve.com
PPT Medical Device Development Tools FDA CDRH Pilot Program Medical Device Development Tools Fda Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing innovative medical device. Food and drug administration (fda) to. Using this program, the fda qualifies tools that medical. Medical Device Development Tools Fda.
From www.cognidox.com
Planning Your Medical Device Design & Development Medical Device Development Tools Fda Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. Food and drug administration (fda) to. The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. Ai & machine learningdedicated dev teams The medical device development tool (mddt) program is a way for the u.s.. Medical Device Development Tools Fda.
From www.slideserve.com
PPT Medical Device Development Tools FDA CDRH Pilot Program Medical Device Development Tools Fda The medical device development tools (mddt) program is a formal cdrh program that encourages stakeholders to propose and. The medical device development tool (mddt) program is a way for the u.s. The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing innovative medical device. Using this program, the fda qualifies. Medical Device Development Tools Fda.
From www.slideserve.com
PPT Medical Device Development Tools FDA CDRH Pilot Program Medical Device Development Tools Fda Ai & machine learningdedicated dev teams The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. Food and drug administration (fda) to. Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. The medical device development tools (mddt) program is a formal cdrh program that. Medical Device Development Tools Fda.
From www.slideteam.net
Six Months Medical Device Prototype Development Roadmap With FDA Medical Device Development Tools Fda The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. Food and drug administration (fda) to. The. Medical Device Development Tools Fda.
From www.slideserve.com
PPT Medical Device Development Tools FDA CDRH Pilot Program Medical Device Development Tools Fda The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. The medical device development tool (mddt) program is a way for the u.s. Food and drug administration (fda) to. Using this program, the fda qualifies. Medical Device Development Tools Fda.
From 1technation.com
The FDA Qualifies New Cybersecurity Medical Device Development Tool Medical Device Development Tools Fda Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. Ai & machine learningdedicated dev teams The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. The medical device development tools (mddt) program is a formal cdrh program that encourages. Medical Device Development Tools Fda.
From www.slideserve.com
PPT Medical Device Development Tools FDA CDRH Pilot Program Medical Device Development Tools Fda The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing innovative medical device. The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. The medical device development tool (mddt) program is a way for the u.s. Ai & machine learningdedicated dev teams. Medical Device Development Tools Fda.
From www.slideteam.net
Five Years Medical Device Prototype Development Roadmap With FDA Medical Device Development Tools Fda The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. The medical device development tool (mddt) program is a way for the u.s. The medical device development tools (mddt) program is a formal cdrh program that encourages stakeholders to propose and. Ai & machine learningdedicated dev teams Food and drug administration (fda) to.. Medical Device Development Tools Fda.
From www.slideserve.com
PPT Medical Device Development Tools FDA CDRH Pilot Program Medical Device Development Tools Fda The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing innovative medical device. Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. The medical device development tool (mddt) program is a way for the u.s. Using this. Medical Device Development Tools Fda.
From operonstrategist.com
US FDA 21 CFR 820.30 (Design Controls For Medical Devices) Operon Medical Device Development Tools Fda Ai & machine learningdedicated dev teams The medical device development tool (mddt) program is a way for the u.s. The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing innovative medical device. Section. Medical Device Development Tools Fda.
From paradigmit.com
Human Factors Considerations in Medical Device Development and FDA Medical Device Development Tools Fda Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. Food and drug administration (fda) to. Ai & machine learningdedicated dev teams The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. The medical device development tools (mddt) program is a formal cdrh program that. Medical Device Development Tools Fda.
From www.slideserve.com
PPT Medical Device Development Tools FDA CDRH Pilot Program Medical Device Development Tools Fda The medical device development tools (mddt) program is a formal cdrh program that encourages stakeholders to propose and. The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing innovative medical device. The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. Using this. Medical Device Development Tools Fda.
From sunstonepilot.com
FDA Software Guidances and the IEC 62304 Software Standard Sunstone Medical Device Development Tools Fda The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing innovative medical device. Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. Ai. Medical Device Development Tools Fda.
From www.mastertrial.com
FDA Regulation for Medical Devices Mastertrial Medical Device Development Tools Fda The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. The medical device development tool (mddt) program is a way for the u.s. Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. Section 3011 of the 21st century cures act (cures act)2 added. Medical Device Development Tools Fda.
From www.mastertrial.com
FDA Medical Device Development Tools (MDDT) Program Mastertrial Medical Device Development Tools Fda The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing innovative medical device. Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts),. Medical Device Development Tools Fda.
From www.meditrial.net
US FDA introduces new Medical Device Development Tools for sponsors Medical Device Development Tools Fda The medical device development tool (mddt) program is a way for the u.s. Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. Using this program, the fda qualifies tools. Medical Device Development Tools Fda.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Medical Device Development Tools Fda The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing innovative medical device. Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts),. Medical Device Development Tools Fda.
From starfishmedical.com
Qualifying Medical Device Development Tools (MDDT) StarFish Medical Medical Device Development Tools Fda Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. Food and drug administration (fda) to. The medical device development tool (mddt) program is a way for the u.s. The us food and drug administration (fda) announced a new medical device development tools (mddt) program on. The medical device development tools (mddt). Medical Device Development Tools Fda.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Device Development Tools Fda Food and drug administration (fda) to. Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. The program aims to provide mddt developers and medical device sponsors with a mechanism. Medical Device Development Tools Fda.
From starfishmedical.com
Medical device contract manufacturing StarFish Medical Medical Device Development Tools Fda Food and drug administration (fda) to. The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing innovative medical device. Ai & machine learningdedicated dev teams Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. Using this program,. Medical Device Development Tools Fda.
From www.vrogue.co
A Guide To Fda Design Controls For Your Medical Devic vrogue.co Medical Device Development Tools Fda The medical device development tool (mddt) program is a way for the u.s. Food and drug administration (fda) to. The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing innovative medical device. Ai & machine learningdedicated dev teams The medical device development tools (mddt) program is a formal cdrh program. Medical Device Development Tools Fda.
From www.kolabtree.com
A guide to FDA Design Controls for your medical device Medical Device Development Tools Fda The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. Ai & machine learningdedicated dev teams The medical device development tools (mddt) program is a formal cdrh program that encourages. Medical Device Development Tools Fda.
From template.mapadapalavra.ba.gov.br
Medical Device Design And Development Plan Template Medical Device Development Tools Fda The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. Food and. Medical Device Development Tools Fda.
From www.iqvia.com
FDA Publishes Approved List of AI/MLenabled Medical Devices IQVIA Medical Device Development Tools Fda The medical device development tool (mddt) program is a way for the u.s. Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. The medical device development tools (mddt) program is a formal cdrh. Medical Device Development Tools Fda.
From www.clinicalpathwaysresearch.com
FDA Medical Device Development Tool Program Guidance — Clinical Pathways Medical Device Development Tools Fda The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing innovative medical device. Food and drug administration (fda) to. Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. The medical device development tool (mddt) program is a way for the u.s.. Medical Device Development Tools Fda.
From www.slideserve.com
PPT Medical Device Development Tools FDA CDRH Pilot Program Medical Device Development Tools Fda The medical device development tools (mddt) program is a formal cdrh program that encourages stakeholders to propose and. The medical device development tool (mddt) program is a way for the u.s. Using this program, the fda qualifies tools that medical device sponsors can use in developing and evaluating medical. The program aims to provide mddt developers and medical device sponsors. Medical Device Development Tools Fda.
From www.greenlight.guru
ISO 13485 and FDA QSR A StepbyStep Guide to Complying with Medical Medical Device Development Tools Fda Section 3011 of the 21st century cures act (cures act)2 added new section 507, qualification of drug3 development tools (ddts), to the. The program aims to provide mddt developers and medical device sponsors with a mechanism for discussing early concepts. The fda center for devices and radiological health (cdrh) has announced new funding opportunities to support small businesses in developing. Medical Device Development Tools Fda.