Strong Base Reaction . Any acid that dissociates 100% into ions is called a strong acid. Explain the ph scale, and convert ph and concentration of. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. This leads to complete neutralization, forming a. Strong acid and base react completely and neutralize each. If it does not dissociate 100%, it is a weak acid. Strong acid with strong base : This is called a neutralization reaction and looks like this: An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): The acidity or basicity of an aqueous solution is described quantitatively using. Mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. Give the names and formulas of some strong acids and bases.
from www.chegg.com
Strong acid with strong base : This is called a neutralization reaction and looks like this: Strong acid and base react completely and neutralize each. An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): Give the names and formulas of some strong acids and bases. Any acid that dissociates 100% into ions is called a strong acid. Mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. This leads to complete neutralization, forming a. The acidity or basicity of an aqueous solution is described quantitatively using. Explain the ph scale, and convert ph and concentration of.
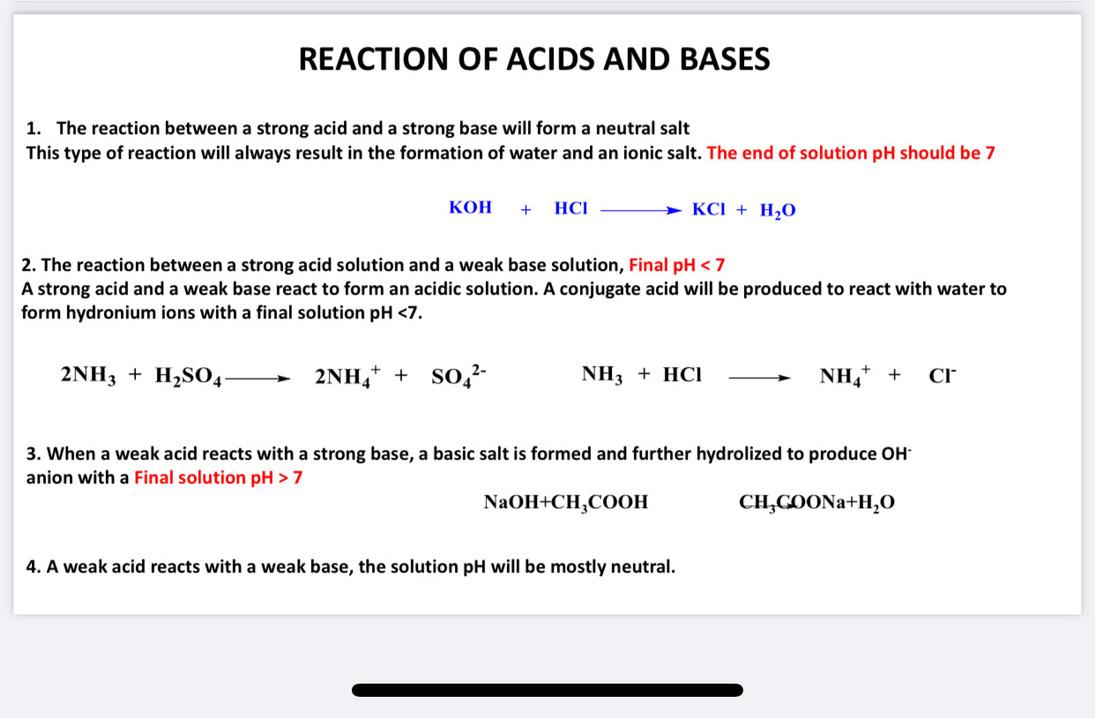
Solved REACTION OF ACIDS AND BASES 1. The reaction between a
Strong Base Reaction Strong acid with strong base : If it does not dissociate 100%, it is a weak acid. Explain the ph scale, and convert ph and concentration of. Any acid that dissociates 100% into ions is called a strong acid. Strong acid with strong base : Give the names and formulas of some strong acids and bases. The acidity or basicity of an aqueous solution is described quantitatively using. This leads to complete neutralization, forming a. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): Strong acid and base react completely and neutralize each. This is called a neutralization reaction and looks like this:
From docslib.org
Elimination Reactions Heating an Alkyl Halide with a Strong Base Causes Strong Base Reaction If it does not dissociate 100%, it is a weak acid. Strong acid with strong base : Mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): The reaction of. Strong Base Reaction.
From stock.adobe.com
Strong acid and strong base reaction. Strengths of acids and bases Strong Base Reaction Strong acid with strong base : Explain the ph scale, and convert ph and concentration of. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. If it does not dissociate 100%, it is a weak acid. This leads to complete neutralization, forming a. An example would be the reaction. Strong Base Reaction.
From app.jove.com
Crossed Aldol Reaction Using Strong Bases Directed Aldol Reaction Strong Base Reaction Mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. Strong acid and base react completely and neutralize each. This is called a neutralization reaction and looks like this: Strong acid with strong base : Explain the ph scale, and convert ph and concentration of. This leads to complete neutralization,. Strong Base Reaction.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free Strong Base Reaction The acidity or basicity of an aqueous solution is described quantitatively using. Strong acid with strong base : Explain the ph scale, and convert ph and concentration of. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. An example would be the reaction between the strong acid hcl (hydrochloric. Strong Base Reaction.
From www.slideserve.com
PPT CHAPTER 10 Reactions in Aqueous Solutions I Acids, Bases & Salts Strong Base Reaction The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Explain the ph scale, and convert ph and concentration of. Strong acid with strong base : Strong acid and base react completely and neutralize each. If it does not dissociate 100%, it is a weak acid. This leads to complete. Strong Base Reaction.
From www.slideserve.com
PPT Water The Universal Solvent PowerPoint Presentation, free Strong Base Reaction This leads to complete neutralization, forming a. This is called a neutralization reaction and looks like this: Any acid that dissociates 100% into ions is called a strong acid. Mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. The reaction of a strong acid with a strong base is. Strong Base Reaction.
From www.youtube.com
Reaction weak acid Strong Base YouTube Strong Base Reaction This is called a neutralization reaction and looks like this: Strong acid with strong base : Explain the ph scale, and convert ph and concentration of. If it does not dissociate 100%, it is a weak acid. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Mixing equal amounts. Strong Base Reaction.
From www.teachoo.com
Examples of Weak Base (5 Examples with Images) Teachoo Chemistry Strong Base Reaction This leads to complete neutralization, forming a. If it does not dissociate 100%, it is a weak acid. Strong acid with strong base : The acidity or basicity of an aqueous solution is described quantitatively using. This is called a neutralization reaction and looks like this: Strong acid and base react completely and neutralize each. Any acid that dissociates 100%. Strong Base Reaction.
From www.organicchemistrytutor.com
E2 Reactions — Organic Chemistry Tutor Strong Base Reaction An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): If it does not dissociate 100%, it is a weak acid. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. The acidity or basicity of an aqueous solution is. Strong Base Reaction.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID Strong Base Reaction Explain the ph scale, and convert ph and concentration of. Mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. Strong acid with strong base : Give the names and formulas of some strong acids and bases. Any acid that dissociates 100% into ions is called a strong acid. Strong. Strong Base Reaction.
From www.slideserve.com
PPT Chapter 4 Reactions in Aqueous Solution PowerPoint Presentation Strong Base Reaction This is called a neutralization reaction and looks like this: Strong acid with strong base : The acidity or basicity of an aqueous solution is described quantitatively using. An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): Any acid that dissociates 100% into ions is called a strong acid.. Strong Base Reaction.
From stock.adobe.com
Strong acid and strong base reaction. Strengths of acids and bases Strong Base Reaction Strong acid and base react completely and neutralize each. Any acid that dissociates 100% into ions is called a strong acid. An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): If it does not dissociate 100%, it is a weak acid. The reaction of a strong acid with a. Strong Base Reaction.
From www.khanacademy.org
Acidbase reactions (practice) Khan Academy Strong Base Reaction Any acid that dissociates 100% into ions is called a strong acid. If it does not dissociate 100%, it is a weak acid. Give the names and formulas of some strong acids and bases. The acidity or basicity of an aqueous solution is described quantitatively using. The reaction of a strong acid with a strong base is a neutralization reaction,. Strong Base Reaction.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID Strong Base Reaction The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Strong acid with strong base : The acidity or basicity of an aqueous solution is described quantitatively using. Any acid that dissociates 100% into ions is called a strong acid. An example would be the reaction between the strong acid. Strong Base Reaction.
From www.youtube.com
Weak acidstrong base reactions Acids and bases AP Chemistry Khan Strong Base Reaction The acidity or basicity of an aqueous solution is described quantitatively using. Strong acid with strong base : An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): Any acid that dissociates 100% into ions is called a strong acid. Mixing equal amounts of a strong acid with a strong. Strong Base Reaction.
From www.teachoo.com
List of Strong Bases 7+ Examples Chemistry Teachoo Strong Base Reaction An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): This leads to complete neutralization, forming a. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. Mixing equal amounts of a strong acid with a strong base also produces. Strong Base Reaction.
From www.chegg.com
Solved REACTION OF ACIDS AND BASES 1. The reaction between a Strong Base Reaction Strong acid with strong base : An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): This is called a neutralization reaction and looks like this: Explain the ph scale, and convert ph and concentration of. The reaction of a strong acid with a strong base is a neutralization reaction,. Strong Base Reaction.
From www.slideserve.com
PPT Chemical Reactions in Aqueous Solutions PowerPoint Presentation Strong Base Reaction This is called a neutralization reaction and looks like this: Strong acid with strong base : Any acid that dissociates 100% into ions is called a strong acid. Strong acid and base react completely and neutralize each. Mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. If it does. Strong Base Reaction.
From worksheets.clipart-library.com
Strong acidstrong base reactions Worksheets Library Strong Base Reaction Explain the ph scale, and convert ph and concentration of. An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): This is called a neutralization reaction and looks like this: Mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution.. Strong Base Reaction.
From www.khanacademy.org
Acidbase reactions (practice) Khan Academy Strong Base Reaction An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): Give the names and formulas of some strong acids and bases. Mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. This leads to complete neutralization, forming a. If it. Strong Base Reaction.
From www.slideserve.com
PPT Acid and Base Reactions PowerPoint Presentation, free download Strong Base Reaction Explain the ph scale, and convert ph and concentration of. The acidity or basicity of an aqueous solution is described quantitatively using. Mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. Any acid that dissociates 100% into ions is called a strong acid. This leads to complete neutralization, forming. Strong Base Reaction.
From masterorganicchemistry.com
Organometallics Are Strong Bases — Master Organic Chemistry Strong Base Reaction The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. The acidity or basicity of an aqueous solution is described quantitatively using. Give the names and formulas of some strong acids and bases. An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base. Strong Base Reaction.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Strong Base Reaction Strong acid with strong base : Strong acid and base react completely and neutralize each. Explain the ph scale, and convert ph and concentration of. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. This is called a neutralization reaction and looks like this: The acidity or basicity of. Strong Base Reaction.
From www.chemistrylearner.com
AcidBase Reaction Definition, Examples, and Uses Strong Base Reaction If it does not dissociate 100%, it is a weak acid. Any acid that dissociates 100% into ions is called a strong acid. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. This leads to complete neutralization, forming a. This is called a neutralization reaction and looks like this:. Strong Base Reaction.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases Strong Base Reaction This is called a neutralization reaction and looks like this: Strong acid and base react completely and neutralize each. Strong acid with strong base : If it does not dissociate 100%, it is a weak acid. Explain the ph scale, and convert ph and concentration of. An example would be the reaction between the strong acid hcl (hydrochloric acid) with. Strong Base Reaction.
From www.slideserve.com
PPT Acids and bases, salts and solutions PowerPoint Presentation Strong Base Reaction Strong acid with strong base : An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): Give the names and formulas of some strong acids and bases. Strong acid and base react completely and neutralize each. This leads to complete neutralization, forming a. This is called a neutralization reaction and. Strong Base Reaction.
From avopix.com
Strong acid and strong base reaction. Strengths Royalty Free Stock Strong Base Reaction Mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. Strong acid with strong base : Strong acid and base react completely and neutralize each. If it does not dissociate 100%, it is a weak acid. Explain the ph scale, and convert ph and concentration of. An example would be. Strong Base Reaction.
From www.slideserve.com
PPT AcidBase Reactions TITRATION PowerPoint Presentation, free Strong Base Reaction Strong acid with strong base : Explain the ph scale, and convert ph and concentration of. Give the names and formulas of some strong acids and bases. This leads to complete neutralization, forming a. Any acid that dissociates 100% into ions is called a strong acid. This is called a neutralization reaction and looks like this: If it does not. Strong Base Reaction.
From www.chemistrysteps.com
SN1 SN2 E1 E2 How to choose the coorect mechanism Strong Base Reaction Strong acid and base react completely and neutralize each. An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): Explain the ph scale, and convert ph and concentration of. This is called a neutralization reaction and looks like this: The reaction of a strong acid with a strong base is. Strong Base Reaction.
From www.youtube.com
If the heat of neutralization for a strong acid base reaction is `57 Strong Base Reaction The acidity or basicity of an aqueous solution is described quantitatively using. An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): This is called a neutralization reaction and looks like this: The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus. Strong Base Reaction.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID Strong Base Reaction This leads to complete neutralization, forming a. Explain the ph scale, and convert ph and concentration of. Strong acid with strong base : The acidity or basicity of an aqueous solution is described quantitatively using. If it does not dissociate 100%, it is a weak acid. Mixing equal amounts of a strong acid with a strong base also produces a. Strong Base Reaction.
From www.slideserve.com
PPT Alcohols PowerPoint Presentation, free download ID2426063 Strong Base Reaction The acidity or basicity of an aqueous solution is described quantitatively using. Any acid that dissociates 100% into ions is called a strong acid. Give the names and formulas of some strong acids and bases. Mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. Strong acid with strong base. Strong Base Reaction.
From www.masterorganicchemistry.com
Walkthrough of Acid Base Reactions (2) Basicity Master Organic Chemistry Strong Base Reaction The acidity or basicity of an aqueous solution is described quantitatively using. Explain the ph scale, and convert ph and concentration of. This is called a neutralization reaction and looks like this: This leads to complete neutralization, forming a. Strong acid with strong base : Strong acid and base react completely and neutralize each. Any acid that dissociates 100% into. Strong Base Reaction.
From www.youtube.com
Strong acidstrong base reactions Acids and bases AP Chemistry Strong Base Reaction If it does not dissociate 100%, it is a weak acid. Strong acid and base react completely and neutralize each. The acidity or basicity of an aqueous solution is described quantitatively using. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt. An example would be the reaction between the. Strong Base Reaction.
From www.slideserve.com
PPT Autoionization of Water PowerPoint Presentation, free download Strong Base Reaction An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): Mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. Strong acid and base react completely and neutralize each. The reaction of a strong acid with a strong base is. Strong Base Reaction.