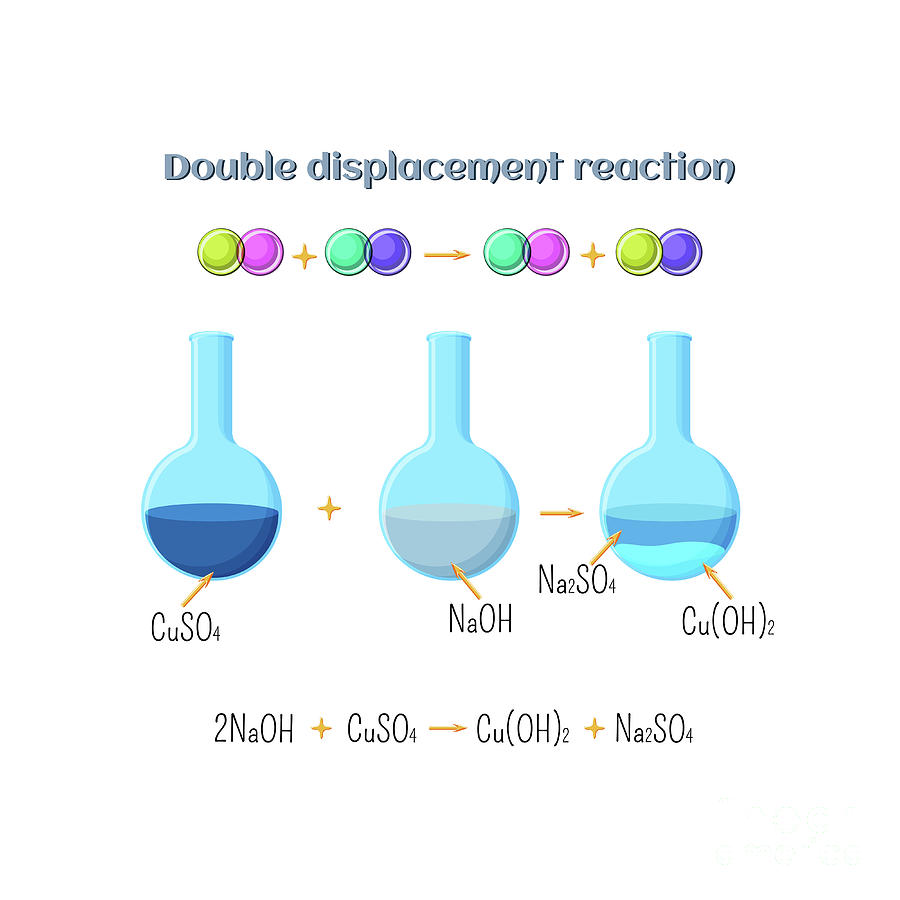
Double Displacement Examples . Both silver nitrate and sodium chloride are ionic compounds. Both reactants dissolve into their ions in aqueous solution. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. What is double displacement reaction? A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds.
from pixels.com
What is double displacement reaction? Both reactants dissolve into their ions in aqueous solution. The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. Both silver nitrate and sodium chloride are ionic compounds. A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules.
Double Displacement Reaction Photograph by Inna Bigun/science Photo Library
Double Displacement Examples A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. Both reactants dissolve into their ions in aqueous solution. A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. Both silver nitrate and sodium chloride are ionic compounds. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. What is double displacement reaction?
From sciencenotes.org
Double Replacement Reaction Definition and Examples Double Displacement Examples \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. Both reactants dissolve into their ions in aqueous solution. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. Both silver nitrate and sodium chloride are ionic compounds.. Double Displacement Examples.
From www.slideserve.com
PPT Double Displacement Reactions PowerPoint Presentation, free Double Displacement Examples What is double displacement reaction? \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. A double displacement reaction, also known as a double replacement. Double Displacement Examples.
From mayang.dilihatya.com
Double Displacement Reaction Definition Chemistry information online Double Displacement Examples A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride. Double Displacement Examples.
From byjus.com
Give examples of Double displacement reactions. Double Displacement Examples An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. What is double displacement reaction? Both reactants dissolve into their ions in aqueous solution. Both silver nitrate and sodium chloride are ionic compounds. A double displacement reaction is a type of chemical reaction in which two. Double Displacement Examples.
From edurev.in
What is double displacement reaction? EduRev Class 10 Question Double Displacement Examples The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. Both silver nitrate and sodium chloride are ionic compounds. What is double displacement reaction? \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic. Double Displacement Examples.
From pencilartdrawingseasydisney.blogspot.com
balancing double displacement reactions pencilArtDrawingsEasyDisney Double Displacement Examples \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. What is double displacement reaction? An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. The double displacement reaction occurs in an aqueous. Double Displacement Examples.
From study.com
How to Identify a Double Displacement Reaction Chemistry Double Displacement Examples What is double displacement reaction? Both reactants dissolve into their ions in aqueous solution. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. Both silver nitrate and sodium chloride are ionic compounds. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. The double displacement reaction occurs in an aqueous. Double Displacement Examples.
From www.youtube.com
DOUBLE DISPLACEMENT REACTIONS YouTube Double Displacement Examples The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. \[\ce{ab}. Double Displacement Examples.
From www.slideserve.com
PPT Chemical Changes PowerPoint Presentation ID3575835 Double Displacement Examples Both reactants dissolve into their ions in aqueous solution. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. An example of a double replacement reaction. Double Displacement Examples.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID497232 Double Displacement Examples Both reactants dissolve into their ions in aqueous solution. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. What is double displacement reaction? A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. A double displacement reaction, also known as. Double Displacement Examples.
From www.slideserve.com
PPT REACTION TYPES PowerPoint Presentation, free download ID4695656 Double Displacement Examples Both reactants dissolve into their ions in aqueous solution. Both silver nitrate and sodium chloride are ionic compounds. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. \[\ce{ab}. Double Displacement Examples.
From www.slideserve.com
PPT (2.3) TYPES OF CHEMICAL REACTIONS PowerPoint Presentation, free Double Displacement Examples \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. Both reactants dissolve into their ions in aqueous solution. A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. Both silver nitrate and sodium chloride. Double Displacement Examples.
From www.thoughtco.com
Double Displacement Reaction Definition and Examples Double Displacement Examples The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. Both silver nitrate and sodium chloride are ionic compounds. A double displacement reaction is a type of chemical reaction. Double Displacement Examples.
From classdbfloyd.z19.web.core.windows.net
Double Displacement Reaction Worksheets Double Displacement Examples Both reactants dissolve into their ions in aqueous solution. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. Both silver nitrate and sodium chloride are ionic compounds. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. \[\ce{ab}. Double Displacement Examples.
From www.youtube.com
Double Displacement Reactions with Examples Chemical Reactions and Double Displacement Examples A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. What is double displacement reaction? A double displacement reaction is a type of. Double Displacement Examples.
From www.slideserve.com
PPT Double Displacement Reactions PowerPoint Presentation, free Double Displacement Examples A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. Both reactants dissolve into their ions in aqueous solution. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. What is double displacement. Double Displacement Examples.
From www.slideserve.com
PPT Double Displacement Reactions PowerPoint Presentation ID40745 Double Displacement Examples \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. What is double displacement reaction? A double displacement reaction is a type of. Double Displacement Examples.
From www.slideserve.com
PPT Double Displacement Reactions PowerPoint Presentation, free Double Displacement Examples A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. Both silver nitrate and sodium chloride are ionic compounds. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. What. Double Displacement Examples.
From education-portal.com
Double Displacement Reaction Definition & Examples Video & Lesson Double Displacement Examples A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. Both reactants dissolve into their ions in aqueous solution. Both. Double Displacement Examples.
From www.youtube.com
Chemical Reaction and equation (Double Displacement Reaction) class Double Displacement Examples Both silver nitrate and sodium chloride are ionic compounds. A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. A double displacement reaction, also known. Double Displacement Examples.
From www.showme.com
Double Displacement Reaction Tutorial Science ShowMe Double Displacement Examples An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. Both silver nitrate and sodium chloride are ionic compounds. The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. A double displacement reaction, also known as a double. Double Displacement Examples.
From byjus.com
write any three differences between displacement and double Double Displacement Examples What is double displacement reaction? A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. An example of a double. Double Displacement Examples.
From pixels.com
Double Displacement Reaction Photograph by Inna Bigun/science Photo Library Double Displacement Examples \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. What is double displacement reaction? A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. Both silver nitrate and sodium chloride are ionic compounds. The. Double Displacement Examples.
From www.teachoo.com
Double Displacement Reaction Definition, Examples, Types Teachoo Double Displacement Examples An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. Both silver nitrate and sodium chloride are ionic compounds. A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. What is double displacement reaction? The. Double Displacement Examples.
From brainly.in
what is double displacement reaction ? give it's 10 examples. Brainly.in Double Displacement Examples A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. An example of a double replacement reaction is the reaction between silver nitrate and sodium. Double Displacement Examples.
From byjus.com
What is a double displacement reaction? Double Displacement Examples An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. Both reactants dissolve into their ions in aqueous solution. Both silver nitrate and sodium chloride are ionic compounds. A. Double Displacement Examples.
From psiberg.com
Double Displacement Reactions A Perfect Give & Take PSIBERG Double Displacement Examples Both silver nitrate and sodium chloride are ionic compounds. The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. Both reactants dissolve into their ions in aqueous solution. What. Double Displacement Examples.
From animalia-life.club
Double Displacement Reaction Examples In Real Life Double Displacement Examples A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. Both reactants dissolve into their ions in aqueous solution. Both silver nitrate and sodium chloride are ionic compounds. The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. \[\ce{ab}. Double Displacement Examples.
From pediaa.com
Difference Between Displacement and Double Displacement Reaction Double Displacement Examples Both silver nitrate and sodium chloride are ionic compounds. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. Both reactants dissolve into their ions in aqueous solution. A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. A double displacement. Double Displacement Examples.
From scienceinfo.com
Double Displacement Reaction Definition, Types, Examples Double Displacement Examples A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. What is double displacement reaction? Both reactants dissolve into their ions in aqueous solution. The double displacement reaction occurs in an. Double Displacement Examples.
From www.chemistrylearner.com
Doublereplacement (Doubledisplacement) Reaction Double Displacement Examples An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. A double displacement reaction is a type of chemical reaction in which two. Double Displacement Examples.
From sites.google.com
Double Displacement Double Displacement Examples What is double displacement reaction? An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. Both reactants dissolve into their ions in aqueous solution. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic. Double Displacement Examples.
From education-portal.com
Double Displacement Reaction Definition & Examples Video & Lesson Double Displacement Examples An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water. Both silver nitrate and sodium chloride are ionic compounds. Both reactants dissolve into their ions in aqueous solution. A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. The double displacement. Double Displacement Examples.
From www.youtube.com
Chemical reactions DOUBLE DISPLACEMENT REACTION YouTube Double Displacement Examples The double displacement reaction occurs in an aqueous solution and usually leads in the formation of a precipitate. \[\ce{ab} + \ce{cd} \rightarrow \ce{ad} +. A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. What is double displacement reaction? Both reactants dissolve into their ions in aqueous. Double Displacement Examples.
From www.slideserve.com
PPT Types of Reactions PowerPoint Presentation, free download ID Double Displacement Examples A double displacement reaction is a type of chemical reaction in which two reactants exchange ions to generate two new molecules. What is double displacement reaction? A double displacement reaction, also known as a double replacement reaction, is a type of chemical reaction that occurs when two ionic compounds. An example of a double replacement reaction is the reaction between. Double Displacement Examples.