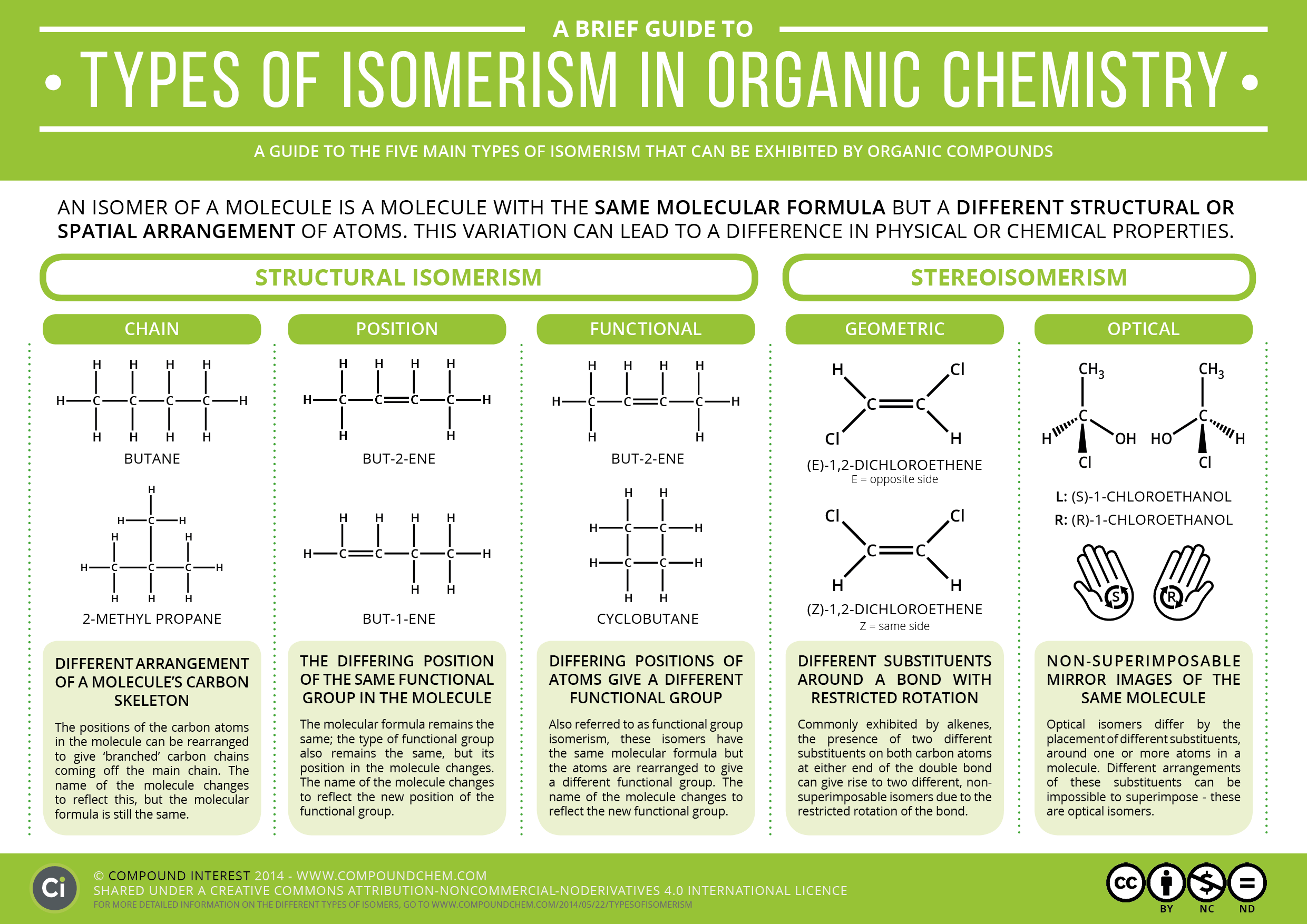
Chain Isomerism Definition With Example . The simplest hydrocarbons like methane, ethane, and propane do not. Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. For example, there are two isomers of butane,. Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. These isomers arise because of the possibility of branching in carbon chains. Chain isomerism is a type of structural isomerism where the isomers have same molecular. While chain isomerism focuses on. Structural isomerism can be further classified as: Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same. In these isomers, the carbon atoms are bonded together in different ways to produce branches. Alkane molecules with four or more carbon atoms have chain isomers.
from www.compoundchem.com
Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. Alkane molecules with four or more carbon atoms have chain isomers. While chain isomerism focuses on. Chain isomerism is a type of structural isomerism where the isomers have same molecular. The simplest hydrocarbons like methane, ethane, and propane do not. Structural isomerism can be further classified as: Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same. Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. For example, there are two isomers of butane,. These isomers arise because of the possibility of branching in carbon chains.
A Brief Guide to Types of Isomerism in Organic Chemistry Compound
Chain Isomerism Definition With Example While chain isomerism focuses on. For example, there are two isomers of butane,. Alkane molecules with four or more carbon atoms have chain isomers. These isomers arise because of the possibility of branching in carbon chains. The simplest hydrocarbons like methane, ethane, and propane do not. Chain isomerism is a type of structural isomerism where the isomers have same molecular. Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. While chain isomerism focuses on. Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same. In these isomers, the carbon atoms are bonded together in different ways to produce branches. Structural isomerism can be further classified as:
From www.slideserve.com
PPT Isomerism PowerPoint Presentation, free download ID2319579 Chain Isomerism Definition With Example The simplest hydrocarbons like methane, ethane, and propane do not. Structural isomerism can be further classified as: Alkane molecules with four or more carbon atoms have chain isomers. Chain isomerism is a type of structural isomerism where the isomers have same molecular. In these isomers, the carbon atoms are bonded together in different ways to produce branches. Chain isomerism and. Chain Isomerism Definition With Example.
From xkldase.edu.vn
Details 156+ example of ring chain isomerism super hot xkldase.edu.vn Chain Isomerism Definition With Example Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. Structural isomerism can be further classified as: The simplest hydrocarbons like methane, ethane, and propane do not. For example, there are two isomers of butane,. Alkane molecules with four or. Chain Isomerism Definition With Example.
From www.toppr.com
Explain position isomerism giving one example. Chain Isomerism Definition With Example For example, there are two isomers of butane,. Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same. Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. In these isomers, the carbon atoms are bonded together in different ways to produce branches. Structural. Chain Isomerism Definition With Example.
From www.slideserve.com
PPT ORGANIC CHEMISTRY PowerPoint Presentation, free download ID2413506 Chain Isomerism Definition With Example Alkane molecules with four or more carbon atoms have chain isomers. Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. For example, there are two isomers of butane,. Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. Isomerism, the existence of molecules that have the same numbers of. Chain Isomerism Definition With Example.
From www.aakash.ac.in
Isomerism Definition, Classification, Structural Isomerism Chain Isomerism Definition With Example These isomers arise because of the possibility of branching in carbon chains. For example, there are two isomers of butane,. Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. While chain isomerism focuses on. Structural isomerism can be further classified as: The simplest hydrocarbons like methane, ethane, and propane do not. Chain isomerism. Chain Isomerism Definition With Example.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID4766560 Chain Isomerism Definition With Example In these isomers, the carbon atoms are bonded together in different ways to produce branches. Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. Alkane molecules with four or more carbon atoms have chain isomers. Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence. Chain Isomerism Definition With Example.
From www.slideshare.net
Isomerism class 11 CBSE organic chemistry some basic principles techn… Chain Isomerism Definition With Example For example, there are two isomers of butane,. Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. The simplest hydrocarbons like methane, ethane, and propane do not. While chain isomerism focuses on. Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. Chain isomerism is a type of structural. Chain Isomerism Definition With Example.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 4.1.2 Naming Organic Compounds Chain Isomerism Definition With Example While chain isomerism focuses on. For example, there are two isomers of butane,. Alkane molecules with four or more carbon atoms have chain isomers. Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. Structural isomerism can be further classified as: Isomerism, the existence of molecules that have the same numbers of the same kinds of. Chain Isomerism Definition With Example.
From www.slideshare.net
Isomers and isomerism Chain Isomerism Definition With Example Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. Chain isomerism is a type of structural isomerism where the isomers have same molecular. The simplest hydrocarbons like methane, ethane, and propane do not. Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same.. Chain Isomerism Definition With Example.
From www.youtube.com
Chain Isomerism and easy way of its Identification YouTube Chain Isomerism Definition With Example Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. For example, there are two isomers of butane,. The simplest hydrocarbons like methane, ethane, and propane do not. In these isomers, the carbon atoms are bonded together in different ways to produce branches. Structural isomerism can be further classified as: These isomers arise because of the. Chain Isomerism Definition With Example.
From scienceinfo.com
Isomerism Definition, Types, Examples Chain Isomerism Definition With Example For example, there are two isomers of butane,. The simplest hydrocarbons like methane, ethane, and propane do not. Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same. Also known as chain isomerism, the arrangement of. Chain Isomerism Definition With Example.
From xkldase.edu.vn
Aggregate more than 148 ring chain isomerism example super hot Chain Isomerism Definition With Example Alkane molecules with four or more carbon atoms have chain isomers. Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. These isomers arise because of the possibility of branching in carbon chains. Structural isomerism can be further classified as:. Chain Isomerism Definition With Example.
From www.gbu-presnenskij.ru
Isomerism Definition, Types, Structure, Examples, And FAQs, 43 OFF Chain Isomerism Definition With Example Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same. The simplest hydrocarbons like methane, ethane, and propane do not. Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. For example, there are two isomers of butane,. In these isomers, the carbon atoms are bonded. Chain Isomerism Definition With Example.
From askfilo.com
1. CHAIN ISOMERISM The same molecular formula represents two or more co.. Chain Isomerism Definition With Example Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. Structural isomerism can be further classified as: In these isomers, the carbon atoms are bonded together in different ways to produce branches. Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same. Chain isomerism. Chain Isomerism Definition With Example.
From askfilo.com
erent types of structural isomerism are Chain Isomerism When two or m.. Chain Isomerism Definition With Example While chain isomerism focuses on. In these isomers, the carbon atoms are bonded together in different ways to produce branches. Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same. Structural isomerism can be. Chain Isomerism Definition With Example.
From www.vrogue.co
Isomerism Definition Detailed Explanation Types Examp vrogue.co Chain Isomerism Definition With Example Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. These isomers arise because of the possibility of branching in carbon chains. While chain isomerism focuses on. Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same. The simplest hydrocarbons like methane, ethane, and propane do. Chain Isomerism Definition With Example.
From www.wikidoc.org
Isomer wikidoc Chain Isomerism Definition With Example The simplest hydrocarbons like methane, ethane, and propane do not. Structural isomerism can be further classified as: Chain isomerism is a type of structural isomerism where the isomers have same molecular. In these isomers, the carbon atoms are bonded together in different ways to produce branches. These isomers arise because of the possibility of branching in carbon chains. For example,. Chain Isomerism Definition With Example.
From stock.adobe.com
ภาพประกอบสต็อก In ringchain isomerism, one of the isomers has an open Chain Isomerism Definition With Example Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. Structural isomerism can be further classified as: Chain isomerism is a type of structural isomerism where the isomers have same molecular. While chain isomerism focuses on. Alkane molecules with four or more carbon atoms have chain isomers. These isomers arise because of the possibility. Chain Isomerism Definition With Example.
From xkldase.edu.vn
Details 156+ example of ring chain isomerism super hot xkldase.edu.vn Chain Isomerism Definition With Example While chain isomerism focuses on. Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. Structural isomerism can be further classified as: The simplest hydrocarbons like methane, ethane, and propane do not. For example, there are two isomers of butane,. In these isomers, the carbon atoms are bonded together in different ways to produce. Chain Isomerism Definition With Example.
From www.slideshare.net
Structure of polymer chains Chain Isomerism Definition With Example While chain isomerism focuses on. For example, there are two isomers of butane,. Structural isomerism can be further classified as: Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. In these isomers, the carbon atoms are bonded together in different ways to produce branches. Isomerism, the existence of molecules that have the same numbers of. Chain Isomerism Definition With Example.
From www.slideserve.com
PPT Hydrocarbons Isomers PowerPoint Presentation, free download ID Chain Isomerism Definition With Example For example, there are two isomers of butane,. Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same. Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. Chain isomerism is a type of structural isomerism where the isomers have same molecular. These isomers arise because. Chain Isomerism Definition With Example.
From vova.edu.vn
Top 130+ ring chain isomerism example vova.edu.vn Chain Isomerism Definition With Example These isomers arise because of the possibility of branching in carbon chains. In these isomers, the carbon atoms are bonded together in different ways to produce branches. The simplest hydrocarbons like methane, ethane, and propane do not. Structural isomerism can be further classified as: Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers.. Chain Isomerism Definition With Example.
From www.pinterest.com.mx
Isomerism Types with Examples in 2021 Chemistry notes, Chemistry Chain Isomerism Definition With Example Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. In these isomers, the carbon atoms are bonded together in different ways to produce branches. Alkane molecules with four or more carbon atoms have chain isomers. These isomers arise because of the possibility of branching in carbon chains. The simplest hydrocarbons like methane, ethane, and propane. Chain Isomerism Definition With Example.
From www.compoundchem.com
A Brief Guide to Types of Isomerism in Organic Chemistry Compound Chain Isomerism Definition With Example These isomers arise because of the possibility of branching in carbon chains. For example, there are two isomers of butane,. Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same. While chain isomerism focuses. Chain Isomerism Definition With Example.
From stock.adobe.com
Ilustracja Stock Chain Isomerism is also known as skeletal isomerism Chain Isomerism Definition With Example Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same. Structural isomerism can be further classified as: Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. Chain isomerism is a type of structural isomerism where the isomers have same molecular. Chain isomerism and. Chain Isomerism Definition With Example.
From www.docbrown.info
Structural Isomerism chain positional functional group isomers Chain Isomerism Definition With Example The simplest hydrocarbons like methane, ethane, and propane do not. Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. Alkane molecules with four or more carbon atoms have chain isomers. Structural isomerism can be further classified as: Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence. Chain Isomerism Definition With Example.
From isaacphysics.org
Isaac Physics Chain Isomerism Definition With Example Structural isomerism can be further classified as: Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. Chain isomerism is a type of structural isomerism where the isomers have same molecular. Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same. While chain isomerism. Chain Isomerism Definition With Example.
From www.slideshare.net
Isomers and isomerism Chain Isomerism Definition With Example Structural isomerism can be further classified as: While chain isomerism focuses on. Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. Chain isomerism is a type of structural isomerism where the isomers have same molecular. In these isomers, the carbon atoms are bonded together in different ways to produce branches. The simplest hydrocarbons. Chain Isomerism Definition With Example.
From saylordotorg.github.io
BranchedChain Alkanes Chain Isomerism Definition With Example Alkane molecules with four or more carbon atoms have chain isomers. In these isomers, the carbon atoms are bonded together in different ways to produce branches. Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. Structural isomerism can be further classified as: Chain isomerism is a type of structural isomerism where the isomers have same. Chain Isomerism Definition With Example.
From www.slideserve.com
PPT Revision Guide Unit 2 PowerPoint Presentation, free download Chain Isomerism Definition With Example These isomers arise because of the possibility of branching in carbon chains. Alkane molecules with four or more carbon atoms have chain isomers. Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. For example, there are two isomers of butane,. The simplest hydrocarbons like methane, ethane, and propane do not. Chain isomerism and. Chain Isomerism Definition With Example.
From xkldase.edu.vn
Details 156+ example of ring chain isomerism super hot xkldase.edu.vn Chain Isomerism Definition With Example Alkane molecules with four or more carbon atoms have chain isomers. While chain isomerism focuses on. For example, there are two isomers of butane,. These isomers arise because of the possibility of branching in carbon chains. Chain isomerism is a type of structural isomerism where the isomers have same molecular. Also known as chain isomerism, the arrangement of the carbon. Chain Isomerism Definition With Example.
From www.slideserve.com
PPT Lecture 2 Organic Chemistry Carbon Chemistry PowerPoint Chain Isomerism Definition With Example For example, there are two isomers of butane,. In these isomers, the carbon atoms are bonded together in different ways to produce branches. Chain isomerism is a type of structural isomerism where the isomers have same molecular. Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. Also known as chain isomerism, the arrangement of the. Chain Isomerism Definition With Example.
From chem.libretexts.org
1.5 Isomerism Chemistry LibreTexts Chain Isomerism Definition With Example Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same. Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. These isomers arise because of the possibility of branching in carbon chains. Alkane molecules with four or more carbon atoms have chain isomers. For. Chain Isomerism Definition With Example.
From www.slideserve.com
PPT Lecture 2 Organic Chemistry Carbon Chemistry PowerPoint Chain Isomerism Definition With Example Also known as chain isomerism, the arrangement of the carbon chain is different for different isomers. For example, there are two isomers of butane,. These isomers arise because of the possibility of branching in carbon chains. While chain isomerism focuses on. The simplest hydrocarbons like methane, ethane, and propane do not. Isomerism, the existence of molecules that have the same. Chain Isomerism Definition With Example.
From chemistnotes.com
Isomerism in Organic Compounds Chemistry Notes Chain Isomerism Definition With Example Alkane molecules with four or more carbon atoms have chain isomers. Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same. Chain isomerism and position isomerism are two important types of isomerism in organic chemistry. In these isomers, the carbon atoms are bonded together in different ways to produce branches.. Chain Isomerism Definition With Example.