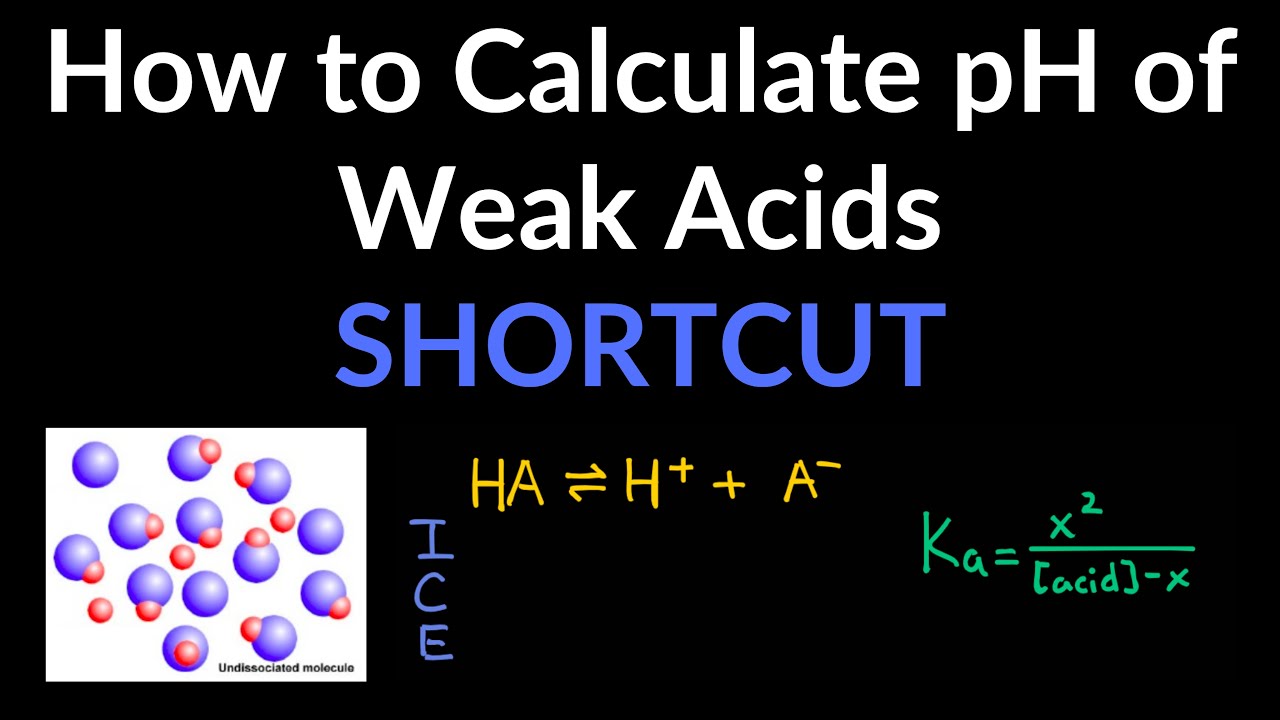
Ice Table Polyprotic Acid . Polyprotic acids are acids that can lose several protons per molecule. The ice table defined x as equal to the bicarbonate ion molarity and the hydronium ion molarity: In order to provide an accurate ph value for polyprotic compounds, this tool takes into account each of the dissociation constants and. An acid that contains more than one ionizable proton is a polyprotic acid. They can be further categorized into diprotic acids and triprotic. Diprotic acids contain two ionizable hydrogen atoms per molecule; Ionization of such acids occurs in two steps. These acids undergo stepwise ionization reactions involving the. An acid that contains more than one ionizable proton is a polyprotic acid. The protons of these acids ionize in steps. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. Using provided information, an ice table for this first step is. H 2 co 3(aq) + h 2 o (l) ⇌ h 3 o+ (aq) + hco 3 −(aq) k a1 = 4.3 × 10 −7.
from www.youtube.com
H 2 co 3(aq) + h 2 o (l) ⇌ h 3 o+ (aq) + hco 3 −(aq) k a1 = 4.3 × 10 −7. Polyprotic acids are acids that can lose several protons per molecule. An acid that contains more than one ionizable proton is a polyprotic acid. These acids undergo stepwise ionization reactions involving the. They can be further categorized into diprotic acids and triprotic. In order to provide an accurate ph value for polyprotic compounds, this tool takes into account each of the dissociation constants and. The protons of these acids ionize in steps. An acid that contains more than one ionizable proton is a polyprotic acid. Diprotic acids contain two ionizable hydrogen atoms per molecule; This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid.
Shortcut to Calculate pH of Weak Acids w/ Examples, Practice Problem
Ice Table Polyprotic Acid H 2 co 3(aq) + h 2 o (l) ⇌ h 3 o+ (aq) + hco 3 −(aq) k a1 = 4.3 × 10 −7. In order to provide an accurate ph value for polyprotic compounds, this tool takes into account each of the dissociation constants and. Ionization of such acids occurs in two steps. H 2 co 3(aq) + h 2 o (l) ⇌ h 3 o+ (aq) + hco 3 −(aq) k a1 = 4.3 × 10 −7. The protons of these acids ionize in steps. Diprotic acids contain two ionizable hydrogen atoms per molecule; This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. Polyprotic acids are acids that can lose several protons per molecule. An acid that contains more than one ionizable proton is a polyprotic acid. They can be further categorized into diprotic acids and triprotic. An acid that contains more than one ionizable proton is a polyprotic acid. The ice table defined x as equal to the bicarbonate ion molarity and the hydronium ion molarity: These acids undergo stepwise ionization reactions involving the. Using provided information, an ice table for this first step is.
From www.youtube.com
Shortcut to Calculate pH of Weak Acids w/ Examples, Practice Problem Ice Table Polyprotic Acid They can be further categorized into diprotic acids and triprotic. Using provided information, an ice table for this first step is. Polyprotic acids are acids that can lose several protons per molecule. Ionization of such acids occurs in two steps. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. In order to provide. Ice Table Polyprotic Acid.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Ice Table Polyprotic Acid An acid that contains more than one ionizable proton is a polyprotic acid. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. The protons of these acids ionize in steps. Ionization of such acids occurs in two steps. They can be further categorized into diprotic acids and triprotic. Diprotic acids contain two ionizable. Ice Table Polyprotic Acid.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Ice Table Polyprotic Acid Using provided information, an ice table for this first step is. Polyprotic acids are acids that can lose several protons per molecule. The ice table defined x as equal to the bicarbonate ion molarity and the hydronium ion molarity: Ionization of such acids occurs in two steps. These acids undergo stepwise ionization reactions involving the. They can be further categorized. Ice Table Polyprotic Acid.
From mungfali.com
Polyprotic Acid Ka Values Ice Table Polyprotic Acid H 2 co 3(aq) + h 2 o (l) ⇌ h 3 o+ (aq) + hco 3 −(aq) k a1 = 4.3 × 10 −7. Ionization of such acids occurs in two steps. An acid that contains more than one ionizable proton is a polyprotic acid. The protons of these acids ionize in steps. These acids undergo stepwise ionization reactions. Ice Table Polyprotic Acid.
From www.slideserve.com
PPT Calculating Percent Ionization PowerPoint Presentation, free Ice Table Polyprotic Acid An acid that contains more than one ionizable proton is a polyprotic acid. In order to provide an accurate ph value for polyprotic compounds, this tool takes into account each of the dissociation constants and. Diprotic acids contain two ionizable hydrogen atoms per molecule; This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid.. Ice Table Polyprotic Acid.
From slideplayer.com
AcidBase Equilibria. ppt download Ice Table Polyprotic Acid An acid that contains more than one ionizable proton is a polyprotic acid. Using provided information, an ice table for this first step is. H 2 co 3(aq) + h 2 o (l) ⇌ h 3 o+ (aq) + hco 3 −(aq) k a1 = 4.3 × 10 −7. This acid base equilibrium video tutorial explains how to calculate the. Ice Table Polyprotic Acid.
From selinanewshurst.blogspot.com
Calculate Ph of Buffer After Adding Naoh Ice Table Polyprotic Acid H 2 co 3(aq) + h 2 o (l) ⇌ h 3 o+ (aq) + hco 3 −(aq) k a1 = 4.3 × 10 −7. Diprotic acids contain two ionizable hydrogen atoms per molecule; These acids undergo stepwise ionization reactions involving the. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. The protons. Ice Table Polyprotic Acid.
From www.chegg.com
Solved Determine the Ka for an acid by constructing an ICE Ice Table Polyprotic Acid In order to provide an accurate ph value for polyprotic compounds, this tool takes into account each of the dissociation constants and. Polyprotic acids are acids that can lose several protons per molecule. Using provided information, an ice table for this first step is. Ionization of such acids occurs in two steps. The ice table defined x as equal to. Ice Table Polyprotic Acid.
From www.bartleby.com
Answered a) Calculate [H3O+] of the following… bartleby Ice Table Polyprotic Acid The ice table defined x as equal to the bicarbonate ion molarity and the hydronium ion molarity: The protons of these acids ionize in steps. An acid that contains more than one ionizable proton is a polyprotic acid. These acids undergo stepwise ionization reactions involving the. Diprotic acids contain two ionizable hydrogen atoms per molecule; H 2 co 3(aq) +. Ice Table Polyprotic Acid.
From www.answersarena.com
[Solved] Determine the Ka for a weak acid by constructing Ice Table Polyprotic Acid In order to provide an accurate ph value for polyprotic compounds, this tool takes into account each of the dissociation constants and. They can be further categorized into diprotic acids and triprotic. An acid that contains more than one ionizable proton is a polyprotic acid. Using provided information, an ice table for this first step is. The ice table defined. Ice Table Polyprotic Acid.
From www.slideserve.com
PPT Chapter 14 PowerPoint Presentation, free download ID5372820 Ice Table Polyprotic Acid Ionization of such acids occurs in two steps. Polyprotic acids are acids that can lose several protons per molecule. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. Using provided information, an ice table for this first step is. H 2 co 3(aq) + h 2 o (l) ⇌ h 3 o+ (aq). Ice Table Polyprotic Acid.
From www.slideserve.com
PPT Chapter 17 Acids and Bases PowerPoint Presentation, free Ice Table Polyprotic Acid The protons of these acids ionize in steps. Ionization of such acids occurs in two steps. In order to provide an accurate ph value for polyprotic compounds, this tool takes into account each of the dissociation constants and. They can be further categorized into diprotic acids and triprotic. Diprotic acids contain two ionizable hydrogen atoms per molecule; These acids undergo. Ice Table Polyprotic Acid.
From www.vrogue.co
Polyprotic Acids Ph Calculation Chemistry Net vrogue.co Ice Table Polyprotic Acid These acids undergo stepwise ionization reactions involving the. An acid that contains more than one ionizable proton is a polyprotic acid. Diprotic acids contain two ionizable hydrogen atoms per molecule; The ice table defined x as equal to the bicarbonate ion molarity and the hydronium ion molarity: Ionization of such acids occurs in two steps. The protons of these acids. Ice Table Polyprotic Acid.
From www.scribd.com
4 Polyprotic Acids and Bases PDF PDF Ice Table Polyprotic Acid These acids undergo stepwise ionization reactions involving the. Using provided information, an ice table for this first step is. H 2 co 3(aq) + h 2 o (l) ⇌ h 3 o+ (aq) + hco 3 −(aq) k a1 = 4.3 × 10 −7. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid.. Ice Table Polyprotic Acid.
From testbook.com
India's No.1 Govt Exam Preparation Site Online Course Ice Table Polyprotic Acid The ice table defined x as equal to the bicarbonate ion molarity and the hydronium ion molarity: Using provided information, an ice table for this first step is. Ionization of such acids occurs in two steps. They can be further categorized into diprotic acids and triprotic. Polyprotic acids are acids that can lose several protons per molecule. An acid that. Ice Table Polyprotic Acid.
From www.slideserve.com
PPT Polyprotic Acids PowerPoint Presentation, free download ID3197268 Ice Table Polyprotic Acid The protons of these acids ionize in steps. They can be further categorized into diprotic acids and triprotic. An acid that contains more than one ionizable proton is a polyprotic acid. An acid that contains more than one ionizable proton is a polyprotic acid. Polyprotic acids are acids that can lose several protons per molecule. This acid base equilibrium video. Ice Table Polyprotic Acid.
From www.numerade.com
SOLVED Problem 2 Sulfuric acid is a polyprotic acid and its Ice Table Polyprotic Acid In order to provide an accurate ph value for polyprotic compounds, this tool takes into account each of the dissociation constants and. The protons of these acids ionize in steps. An acid that contains more than one ionizable proton is a polyprotic acid. They can be further categorized into diprotic acids and triprotic. Using provided information, an ice table for. Ice Table Polyprotic Acid.
From www.numerade.com
SOLVED Report Titration of a Polyprotic Acid A. Data and results from Ice Table Polyprotic Acid An acid that contains more than one ionizable proton is a polyprotic acid. An acid that contains more than one ionizable proton is a polyprotic acid. Using provided information, an ice table for this first step is. Diprotic acids contain two ionizable hydrogen atoms per molecule; This acid base equilibrium video tutorial explains how to calculate the ph of a. Ice Table Polyprotic Acid.
From www.teachmetoscience.com
Polyprotic Acids Ice Table Polyprotic Acid These acids undergo stepwise ionization reactions involving the. The protons of these acids ionize in steps. An acid that contains more than one ionizable proton is a polyprotic acid. They can be further categorized into diprotic acids and triprotic. H 2 co 3(aq) + h 2 o (l) ⇌ h 3 o+ (aq) + hco 3 −(aq) k a1 =. Ice Table Polyprotic Acid.
From www.aiophotoz.com
Polyprotic Acid Titration Curve Images and Photos finder Ice Table Polyprotic Acid These acids undergo stepwise ionization reactions involving the. In order to provide an accurate ph value for polyprotic compounds, this tool takes into account each of the dissociation constants and. Using provided information, an ice table for this first step is. The protons of these acids ionize in steps. They can be further categorized into diprotic acids and triprotic. An. Ice Table Polyprotic Acid.
From marinadesnhwolf.blogspot.com
Identify All of the Polyprotic Acids Below. Ice Table Polyprotic Acid An acid that contains more than one ionizable proton is a polyprotic acid. These acids undergo stepwise ionization reactions involving the. Ionization of such acids occurs in two steps. Using provided information, an ice table for this first step is. In order to provide an accurate ph value for polyprotic compounds, this tool takes into account each of the dissociation. Ice Table Polyprotic Acid.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Ice Table Polyprotic Acid Diprotic acids contain two ionizable hydrogen atoms per molecule; The ice table defined x as equal to the bicarbonate ion molarity and the hydronium ion molarity: In order to provide an accurate ph value for polyprotic compounds, this tool takes into account each of the dissociation constants and. Ionization of such acids occurs in two steps. These acids undergo stepwise. Ice Table Polyprotic Acid.
From general.chemistrysteps.com
pH of a Weak Acid Chemistry Steps Ice Table Polyprotic Acid The protons of these acids ionize in steps. Polyprotic acids are acids that can lose several protons per molecule. An acid that contains more than one ionizable proton is a polyprotic acid. In order to provide an accurate ph value for polyprotic compounds, this tool takes into account each of the dissociation constants and. They can be further categorized into. Ice Table Polyprotic Acid.
From slideplayer.com
Review Predict the products of the following acid base reactions Ice Table Polyprotic Acid In order to provide an accurate ph value for polyprotic compounds, this tool takes into account each of the dissociation constants and. H 2 co 3(aq) + h 2 o (l) ⇌ h 3 o+ (aq) + hco 3 −(aq) k a1 = 4.3 × 10 −7. These acids undergo stepwise ionization reactions involving the. Polyprotic acids are acids that. Ice Table Polyprotic Acid.
From facts.net
19 Fascinating Facts About Polyprotic Acid Ice Table Polyprotic Acid H 2 co 3(aq) + h 2 o (l) ⇌ h 3 o+ (aq) + hco 3 −(aq) k a1 = 4.3 × 10 −7. An acid that contains more than one ionizable proton is a polyprotic acid. These acids undergo stepwise ionization reactions involving the. Diprotic acids contain two ionizable hydrogen atoms per molecule; The ice table defined x. Ice Table Polyprotic Acid.
From www.slideserve.com
PPT ACIDS & BASES PowerPoint Presentation, free download ID3767539 Ice Table Polyprotic Acid They can be further categorized into diprotic acids and triprotic. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. Diprotic acids contain two ionizable hydrogen atoms per molecule; The protons of these acids ionize in steps. Ionization of such acids occurs in two steps. Polyprotic acids are acids that can lose several protons. Ice Table Polyprotic Acid.
From chem.libretexts.org
13.3 Finding the pH of weak Acids, Bases, and Salts Chemistry LibreTexts Ice Table Polyprotic Acid The ice table defined x as equal to the bicarbonate ion molarity and the hydronium ion molarity: These acids undergo stepwise ionization reactions involving the. Diprotic acids contain two ionizable hydrogen atoms per molecule; The protons of these acids ionize in steps. Ionization of such acids occurs in two steps. Using provided information, an ice table for this first step. Ice Table Polyprotic Acid.
From slideplayer.com
Catalyst. ppt download Ice Table Polyprotic Acid These acids undergo stepwise ionization reactions involving the. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. An acid that contains more than one ionizable proton is a polyprotic acid. Polyprotic acids are acids that can lose several protons per molecule. They can be further categorized into diprotic acids and triprotic. Using provided. Ice Table Polyprotic Acid.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Ice Table Polyprotic Acid Using provided information, an ice table for this first step is. Diprotic acids contain two ionizable hydrogen atoms per molecule; In order to provide an accurate ph value for polyprotic compounds, this tool takes into account each of the dissociation constants and. The protons of these acids ionize in steps. An acid that contains more than one ionizable proton is. Ice Table Polyprotic Acid.
From www.dlt.ncssm.edu
TIGER NCSSM Distance Education and Extended Programs Ice Table Polyprotic Acid They can be further categorized into diprotic acids and triprotic. Diprotic acids contain two ionizable hydrogen atoms per molecule; H 2 co 3(aq) + h 2 o (l) ⇌ h 3 o+ (aq) + hco 3 −(aq) k a1 = 4.3 × 10 −7. The protons of these acids ionize in steps. An acid that contains more than one ionizable. Ice Table Polyprotic Acid.
From www.solutioninn.com
[Solved] (A) The solubility of CO 2 (g) in H 2 O a SolutionInn Ice Table Polyprotic Acid Using provided information, an ice table for this first step is. An acid that contains more than one ionizable proton is a polyprotic acid. Diprotic acids contain two ionizable hydrogen atoms per molecule; These acids undergo stepwise ionization reactions involving the. They can be further categorized into diprotic acids and triprotic. H 2 co 3(aq) + h 2 o (l). Ice Table Polyprotic Acid.
From slideplayer.com
Acids and Bases Chapter ppt download Ice Table Polyprotic Acid Polyprotic acids are acids that can lose several protons per molecule. Ionization of such acids occurs in two steps. They can be further categorized into diprotic acids and triprotic. In order to provide an accurate ph value for polyprotic compounds, this tool takes into account each of the dissociation constants and. H 2 co 3(aq) + h 2 o (l). Ice Table Polyprotic Acid.
From www.youtube.com
Acid Base Equilibria Polyprotic Acids Titration. YouTube Ice Table Polyprotic Acid The ice table defined x as equal to the bicarbonate ion molarity and the hydronium ion molarity: An acid that contains more than one ionizable proton is a polyprotic acid. Using provided information, an ice table for this first step is. The protons of these acids ionize in steps. Diprotic acids contain two ionizable hydrogen atoms per molecule; An acid. Ice Table Polyprotic Acid.
From trenahaziim.blogspot.com
13+ Equilibrium Calculations With Ice Tables Worksheet TrenaHaziim Ice Table Polyprotic Acid H 2 co 3(aq) + h 2 o (l) ⇌ h 3 o+ (aq) + hco 3 −(aq) k a1 = 4.3 × 10 −7. An acid that contains more than one ionizable proton is a polyprotic acid. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. Diprotic acids contain two ionizable hydrogen. Ice Table Polyprotic Acid.
From www.chegg.com
Solved The common amino acids are polyprotic acids. All Ice Table Polyprotic Acid Using provided information, an ice table for this first step is. This acid base equilibrium video tutorial explains how to calculate the ph of a polyprotic acid. The protons of these acids ionize in steps. H 2 co 3(aq) + h 2 o (l) ⇌ h 3 o+ (aq) + hco 3 −(aq) k a1 = 4.3 × 10 −7.. Ice Table Polyprotic Acid.