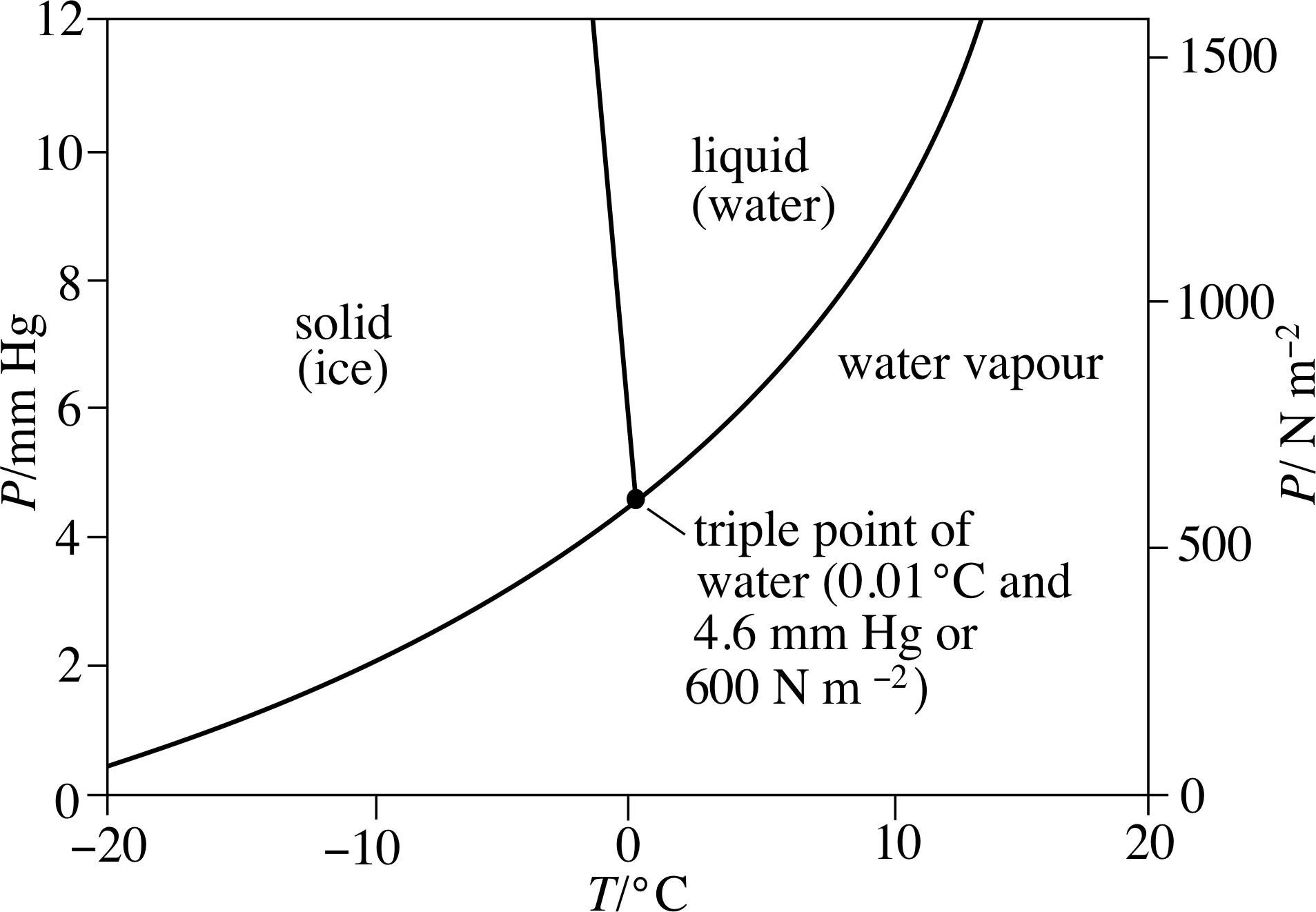
Properties Of Triple Point Of Water . The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. Solid (ice), liquid (water), and gas (water vapor) at a. The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any substance) coexist is. This agreement also sets the size of the kelvin as 1/273.16 of the. People often refer to the triple point temperature of water, but the triple point is really a combination of both. The triple point of water is the unique condition at which water can coexist in all three phases:
from www.physics.brocku.ca
The triple point of water is the unique condition at which water can coexist in all three phases: The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. People often refer to the triple point temperature of water, but the triple point is really a combination of both. This agreement also sets the size of the kelvin as 1/273.16 of the. The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any substance) coexist is. Solid (ice), liquid (water), and gas (water vapor) at a.
PPLATO FLAP PHYS 7.2 Temperature, pressure and the ideal gas laws
Properties Of Triple Point Of Water People often refer to the triple point temperature of water, but the triple point is really a combination of both. The triple point of water is the unique condition at which water can coexist in all three phases: People often refer to the triple point temperature of water, but the triple point is really a combination of both. The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any substance) coexist is. Solid (ice), liquid (water), and gas (water vapor) at a. This agreement also sets the size of the kelvin as 1/273.16 of the.
From pressbooks.online.ucf.edu
12.5 Colligative Properties Chemistry Fundamentals Properties Of Triple Point Of Water People often refer to the triple point temperature of water, but the triple point is really a combination of both. This agreement also sets the size of the kelvin as 1/273.16 of the. The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any. Properties Of Triple Point Of Water.
From byjus.com
two absolute scales A and B have triple pointsof water defined to be Properties Of Triple Point Of Water This agreement also sets the size of the kelvin as 1/273.16 of the. The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any substance) coexist is. People often refer to the triple point temperature of water, but the triple point is really a. Properties Of Triple Point Of Water.
From blog.plover.com
The Universe of Disco A message to the aliens, part 10/23 (temperature) Properties Of Triple Point Of Water The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. This agreement also sets the size of the kelvin as 1/273.16 of the. The triple point of water is the unique condition at which water can coexist in all three phases: Solid (ice),. Properties Of Triple Point Of Water.
From www.nuclear-power.com
Triple Point of Water Definition & Value Properties Of Triple Point Of Water People often refer to the triple point temperature of water, but the triple point is really a combination of both. The triple point of water is the unique condition at which water can coexist in all three phases: Solid (ice), liquid (water), and gas (water vapor) at a. This agreement also sets the size of the kelvin as 1/273.16 of. Properties Of Triple Point Of Water.
From www.youtube.com
Triple Point of Water YouTube Properties Of Triple Point Of Water Solid (ice), liquid (water), and gas (water vapor) at a. The triple point of water is the unique condition at which water can coexist in all three phases: The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. People often refer to the. Properties Of Triple Point Of Water.
From www.youtube.com
Thermal Properties of Matter Triple point Triple point of water Properties Of Triple Point Of Water People often refer to the triple point temperature of water, but the triple point is really a combination of both. The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. This agreement also sets the size of the kelvin as 1/273.16 of the.. Properties Of Triple Point Of Water.
From www.slideserve.com
PPT Zeroth Law of Thermodynamics PowerPoint Presentation, free Properties Of Triple Point Of Water People often refer to the triple point temperature of water, but the triple point is really a combination of both. The triple point of water is the unique condition at which water can coexist in all three phases: The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases. Properties Of Triple Point Of Water.
From www.youtube.com
Triple Point Phase Diagram of water YouTube Properties Of Triple Point Of Water The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any substance) coexist is. This agreement also sets the size of the kelvin as 1/273.16 of the. The triple point of water is the unique condition at which water can coexist in all three. Properties Of Triple Point Of Water.
From www.toppr.com
Two absolute scales A and B have triple points of water defined to be Properties Of Triple Point Of Water This agreement also sets the size of the kelvin as 1/273.16 of the. The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. The triple point of water is the unique condition at which water can coexist in all three phases: People often. Properties Of Triple Point Of Water.
From www.toppr.com
WE5 Two absolute scales X and Y have triple points of water defined Properties Of Triple Point Of Water The triple point of water is the unique condition at which water can coexist in all three phases: The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. Solid (ice), liquid (water), and gas (water vapor) at a. People often refer to the. Properties Of Triple Point Of Water.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii Properties Of Triple Point Of Water The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any substance) coexist is. The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. People often refer. Properties Of Triple Point Of Water.
From www.physics.brocku.ca
PPLATO FLAP PHYS 7.2 Temperature, pressure and the ideal gas laws Properties Of Triple Point Of Water The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any substance) coexist is. People often refer to the triple point temperature of water, but the triple point is really a combination of both. The triple point of a substance is the temperature and. Properties Of Triple Point Of Water.
From www.reddit.com
What if resources (other than water) changed phase? r/Starfield Properties Of Triple Point Of Water The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. The triple point of water is the unique condition at which water can coexist in all three phases: This agreement also sets the size of the kelvin as 1/273.16 of the. The temperature. Properties Of Triple Point Of Water.
From www.wimp.com
The triple point of water Properties Of Triple Point Of Water The triple point of water is the unique condition at which water can coexist in all three phases: Solid (ice), liquid (water), and gas (water vapor) at a. People often refer to the triple point temperature of water, but the triple point is really a combination of both. The triple point of a substance is the temperature and pressure at. Properties Of Triple Point Of Water.
From askfilo.com
The triple point of water corresponds to Filo Properties Of Triple Point Of Water The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. People often refer to the triple point temperature of water, but the triple point is really a combination of both. The temperature and pressure at which the fusion curve, the vaporization curve and. Properties Of Triple Point Of Water.
From www.youtube.com
Triple point of water YouTube Properties Of Triple Point Of Water This agreement also sets the size of the kelvin as 1/273.16 of the. Solid (ice), liquid (water), and gas (water vapor) at a. People often refer to the triple point temperature of water, but the triple point is really a combination of both. The triple point of water is the unique condition at which water can coexist in all three. Properties Of Triple Point Of Water.
From hctechco.com
Triple Point Là Gì? Khái Niệm Và So Sánh Với Critical Point HCTECH Properties Of Triple Point Of Water This agreement also sets the size of the kelvin as 1/273.16 of the. People often refer to the triple point temperature of water, but the triple point is really a combination of both. The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. Properties Of Triple Point Of Water.
From jakobeewtcoller.blogspot.com
Triple Point of Water JakobeewtColler Properties Of Triple Point Of Water The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. The triple point of water is the unique condition at which water can coexist in all three phases: Solid (ice), liquid (water), and gas (water vapor) at a. People often refer to the. Properties Of Triple Point Of Water.
From www.vrogue.co
Phase Diagram Of Water Triple Point vrogue.co Properties Of Triple Point Of Water The triple point of water is the unique condition at which water can coexist in all three phases: The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any substance) coexist is. The triple point of a substance is the temperature and pressure at. Properties Of Triple Point Of Water.
From documents.pub
Bilateral comparison of water triple point cells EURAMET.T Properties Of Triple Point Of Water The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. The triple point of water is the unique condition at which water can coexist in all three phases: People often refer to the triple point temperature of water, but the triple point is. Properties Of Triple Point Of Water.
From www.toppr.com
Two absolute scales A and B have triple points of water defined to be Properties Of Triple Point Of Water The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. The triple point of water is the unique condition at which water can coexist in all three phases: The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation. Properties Of Triple Point Of Water.
From www.zigya.com
The Triple Point of Water Coexistence of Solid Liquid and Gas Properties Of Triple Point Of Water The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any substance) coexist is. The triple point of water is the unique condition at which water can coexist in all three phases: People often refer to the triple point temperature of water, but the. Properties Of Triple Point Of Water.
From www.reddit.com
ELI5 Water triple point? r/explainlikeimfive Properties Of Triple Point Of Water Solid (ice), liquid (water), and gas (water vapor) at a. The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. This agreement also sets the size of the kelvin as 1/273.16 of the. The triple point of water is the unique condition at. Properties Of Triple Point Of Water.
From www.thoughtco.com
Triple Point Definition and Example (Chemistry) Properties Of Triple Point Of Water Solid (ice), liquid (water), and gas (water vapor) at a. The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. This agreement also sets the size of the kelvin as 1/273.16 of the. People often refer to the triple point temperature of water,. Properties Of Triple Point Of Water.
From www.toppr.com
Answer the following(a) The triple point of water is a standard Properties Of Triple Point Of Water People often refer to the triple point temperature of water, but the triple point is really a combination of both. The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any substance) coexist is. The triple point of a substance is the temperature and. Properties Of Triple Point Of Water.
From www.youtube.com
Triple Point of Water Triple Point Diagram Freeze Drying Process Properties Of Triple Point Of Water People often refer to the triple point temperature of water, but the triple point is really a combination of both. Solid (ice), liquid (water), and gas (water vapor) at a. This agreement also sets the size of the kelvin as 1/273.16 of the. The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet. Properties Of Triple Point Of Water.
From www.slideserve.com
PPT Chapter 17 Theory of Gases PowerPoint Presentation, free Properties Of Triple Point Of Water The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any substance) coexist is. This agreement also sets the size of the kelvin as 1/273.16 of the. Solid (ice), liquid (water), and gas (water vapor) at a. The triple point of water is the. Properties Of Triple Point Of Water.
From wirecrafted.com
Understanding the Water Triple Point Diagram Properties Of Triple Point Of Water Solid (ice), liquid (water), and gas (water vapor) at a. The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any substance) coexist is. The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). Properties Of Triple Point Of Water.
From klasmeier.engineer
Water Triple Point Thomas Klasmeier Properties Of Triple Point Of Water This agreement also sets the size of the kelvin as 1/273.16 of the. Solid (ice), liquid (water), and gas (water vapor) at a. People often refer to the triple point temperature of water, but the triple point is really a combination of both. The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet. Properties Of Triple Point Of Water.
From www.pinterest.co.uk
The triple point of water is the only temperature at which water can Properties Of Triple Point Of Water Solid (ice), liquid (water), and gas (water vapor) at a. The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any substance) coexist is. People often refer to the triple point temperature of water, but the triple point is really a combination of both.. Properties Of Triple Point Of Water.
From www.i-ciencias.com
pressure Mezcla de aceite y agua a presión Properties Of Triple Point Of Water The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. The triple point of water is the unique condition at which water can coexist in all three phases: Solid (ice), liquid (water), and gas (water vapor) at a. The temperature and pressure at. Properties Of Triple Point Of Water.
From www.youtube.com
Two absolute scales `A` and `B` have triple points of water defined to Properties Of Triple Point Of Water Solid (ice), liquid (water), and gas (water vapor) at a. The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any substance) coexist is. The triple point of water is the unique condition at which water can coexist in all three phases: The triple. Properties Of Triple Point Of Water.
From sciencenotes.org
Triple Point Definition Triple Point of Water Properties Of Triple Point Of Water The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet and all the three phases of a water (or any substance) coexist is. Solid (ice), liquid (water), and gas (water vapor) at a. The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). Properties Of Triple Point Of Water.
From www.tikalon.com
Tikalon Blog by Dev Gualtieri Properties Of Triple Point Of Water The triple point of water is the unique condition at which water can coexist in all three phases: The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation. Properties Of Triple Point Of Water.
From www.researchgate.net
Phase diagram of water indicating the triple point [33] Download Properties Of Triple Point Of Water Solid (ice), liquid (water), and gas (water vapor) at a. People often refer to the triple point temperature of water, but the triple point is really a combination of both. This agreement also sets the size of the kelvin as 1/273.16 of the. The temperature and pressure at which the fusion curve, the vaporization curve and the sublimation curve meet. Properties Of Triple Point Of Water.