Dilution Of Solutions In The Lab . Dilution is the process of adding more solvent to a solution. Understand how stock solutions are used in the laboratory. The acid must be diluted. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Serial dilutions are used in microbiology to reduce bacterial concentrations. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7. Dilution is the process of weakening or deconcentrating a solution. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. An example of a dilute solution is. Learn how to dilute and concentrate solutions. Solutions can be prepared by diluting a solution of a known higher concentration to. The same can be said of a ratio. Dilution is the addition of solvent,.
from www.chegg.com
The acid must be diluted. Dilution is the process of weakening or deconcentrating a solution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. The same can be said of a ratio. Serial dilutions are used in microbiology to reduce bacterial concentrations. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Solutions can be prepared by diluting a solution of a known higher concentration to. Dilution is the addition of solvent,.
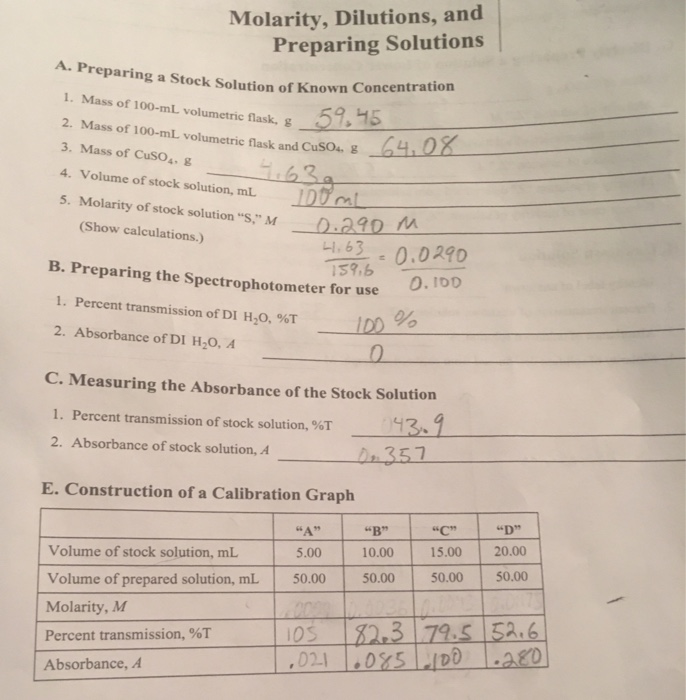
Solved molarity, dilutions, and preparing solutions lab
Dilution Of Solutions In The Lab The same can be said of a ratio. Dilution is the process of adding more solvent to a solution. Dilution is the addition of solvent,. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The same can be said of a ratio. Solutions can be prepared by diluting a solution of a known higher concentration to. The acid must be diluted. Serial dilutions are used in microbiology to reduce bacterial concentrations. Understand how stock solutions are used in the laboratory. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7. An example of a dilute solution is. Dilution is the process of weakening or deconcentrating a solution.
From www.pinterest.com
Solution preparation of 110 dilution formation Molar solution Dilution Of Solutions In The Lab Learn how to dilute and concentrate solutions. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Dilution is the process of weakening or deconcentrating a solution. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7.. Dilution Of Solutions In The Lab.
From chemistryknights.blogspot.com
Chemistry Knights VIDEO DILUTION LAB CALCULATIONS Dilution Of Solutions In The Lab The acid must be diluted. Learn how to dilute and concentrate solutions. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7. The same can be said of a ratio. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. This. Dilution Of Solutions In The Lab.
From www.studypool.com
SOLUTION Dilution laboratory Studypool Dilution Of Solutions In The Lab The same can be said of a ratio. An example of a dilute solution is. Dilution is the process of weakening or deconcentrating a solution. Solutions can be prepared by diluting a solution of a known higher concentration to. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Serial dilutions. Dilution Of Solutions In The Lab.
From loegnwkyw.blob.core.windows.net
Serial Dilution Equation Examples at Keith Rush blog Dilution Of Solutions In The Lab Dilution is the process of adding more solvent to a solution. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7. The acid must be diluted. The same can be said of a ratio. An example of a dilute solution is. Serial dilutions are used in microbiology to reduce bacterial. Dilution Of Solutions In The Lab.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Dilution Of Solutions In The Lab The same can be said of a ratio. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7. An example of a dilute solution is. Serial dilutions are used in microbiology to reduce bacterial concentrations. The acid must be diluted. Solutions can be prepared by diluting a solution of a. Dilution Of Solutions In The Lab.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Of Solutions In The Lab An example of a dilute solution is. Solutions can be prepared by diluting a solution of a known higher concentration to. The same can be said of a ratio. Dilution is the process of weakening or deconcentrating a solution. The acid must be diluted. Dilution is the process of adding more solvent to a solution. Often, a worker will need. Dilution Of Solutions In The Lab.
From studylib.net
Lab The Dilution Solution Dilution Of Solutions In The Lab The same can be said of a ratio. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. Dilution is the addition of solvent,. Learn how. Dilution Of Solutions In The Lab.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Of Solutions In The Lab A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Solutions can be prepared by diluting a solution of a known higher concentration to. Dilution is the process of weakening or deconcentrating a solution. Dilution is the addition of solvent,. Serial dilutions are used in microbiology to reduce bacterial concentrations. A. Dilution Of Solutions In The Lab.
From stock.adobe.com
Scientific experiment diagram show concept of serial dilution for Dilution Of Solutions In The Lab Dilution is the process of adding more solvent to a solution. Dilution is the process of weakening or deconcentrating a solution. Serial dilutions are used in microbiology to reduce bacterial concentrations. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of solvent,. A dilution is made by. Dilution Of Solutions In The Lab.
From ecampusontario.pressbooks.pub
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual Dilution Of Solutions In The Lab Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. The same can be said of a ratio. Often, a worker will need to change the concentration of a solution by. Dilution Of Solutions In The Lab.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilution Of Solutions In The Lab Learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. The acid must be diluted. An example of a dilute solution is. Dilution is the process of adding more solvent to a solution. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. The same. Dilution Of Solutions In The Lab.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Of Solutions In The Lab An example of a dilute solution is. Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Solutions can be prepared by diluting a. Dilution Of Solutions In The Lab.
From microbeonline.com
Serial Dilution Method for Estimating Viable Count of Bacteria Dilution Of Solutions In The Lab Solutions can be prepared by diluting a solution of a known higher concentration to. Dilution is the process of adding more solvent to a solution. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. Dilution is the addition of solvent,. Muriatic acid, another name for. Dilution Of Solutions In The Lab.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Of Solutions In The Lab A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Dilution is the process of adding more solvent to a solution. An example of a dilute solution. Dilution Of Solutions In The Lab.
From mungfali.com
10 Fold Serial Dilution Dilution Of Solutions In The Lab Understand how stock solutions are used in the laboratory. Dilution is the process of weakening or deconcentrating a solution. Solutions can be prepared by diluting a solution of a known higher concentration to. Dilution is the process of adding more solvent to a solution. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate. Dilution Of Solutions In The Lab.
From exytxbygt.blob.core.windows.net
Dilution Definition Literature at Mark Foster blog Dilution Of Solutions In The Lab This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Dilution is the addition of solvent,. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. A dilution is a solution made by adding more solvent to a. Dilution Of Solutions In The Lab.
From www.slideserve.com
PPT Serial Dilution PowerPoint Presentation, free download ID3119682 Dilution Of Solutions In The Lab Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the. Dilution Of Solutions In The Lab.
From www.flinnsci.ca
Preparing and Diluting Solutions Concentration and Absorbance Dilution Of Solutions In The Lab Solutions can be prepared by diluting a solution of a known higher concentration to. The acid must be diluted. Learn how to dilute and concentrate solutions. Dilution is the addition of solvent,. Understand how stock solutions are used in the laboratory. Dilution is the process of adding more solvent to a solution. Dilution is the process of weakening or deconcentrating. Dilution Of Solutions In The Lab.
From spmchemistry.blog.onlinetuition.com.my
Dilution SPM Chemistry Dilution Of Solutions In The Lab Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. Dilution is the addition of solvent,. Understand how stock solutions are used in the laboratory. Dilution. Dilution Of Solutions In The Lab.
From medlabstudyhall.com
Performing Dilutions for Laboratory Analysis Med Lab Study Hall Dilution Of Solutions In The Lab Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7. An example of a dilute solution is. This guide will describe the process for preparing aqueous solutions, including how to calculate the. Dilution Of Solutions In The Lab.
From www.numerade.com
SOLVED It is frequently necessary to perform dilutions of a stock Dilution Of Solutions In The Lab Learn how to dilute and concentrate solutions. Serial dilutions are used in microbiology to reduce bacterial concentrations. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Solutions. Dilution Of Solutions In The Lab.
From exonuljgk.blob.core.windows.net
Cell Culture Dilution Calculator at Leslie Franks blog Dilution Of Solutions In The Lab Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7. Learn how to dilute and concentrate solutions. Dilution is the process of weakening or deconcentrating a solution. The acid must be diluted. An example of a dilute solution is. Solutions can be prepared by diluting a solution of a known. Dilution Of Solutions In The Lab.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Dilution Of Solutions In The Lab An example of a dilute solution is. Solutions can be prepared by diluting a solution of a known higher concentration to. Understand how stock solutions are used in the laboratory. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Often, a worker will need to change. Dilution Of Solutions In The Lab.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution Of Solutions In The Lab Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of solvent,. The same can be said of a ratio. Understand how stock solutions are used in the laboratory. Solutions can be prepared by diluting a solution of a known higher concentration to. The acid must be diluted.. Dilution Of Solutions In The Lab.
From www.slideserve.com
PPT Concentration and Dilution of Solutions PowerPoint Presentation Dilution Of Solutions In The Lab Dilution is the process of adding more solvent to a solution. The acid must be diluted. The same can be said of a ratio. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7. Learn how to dilute and concentrate solutions. An example of a dilute solution is. Solutions can. Dilution Of Solutions In The Lab.
From slideplayer.com
Review of Basic Concepts, Molarity, Solutions and Dilutions ppt download Dilution Of Solutions In The Lab A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. Dilution is the process of adding more solvent to a solution. The same can be said of a ratio. Solutions can be prepared by diluting a solution of a known higher concentration to. Muriatic acid, another. Dilution Of Solutions In The Lab.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Of Solutions In The Lab An example of a dilute solution is. Dilution is the addition of solvent,. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Dilution is the process. Dilution Of Solutions In The Lab.
From www.pinterest.se
Dilution when solvent is added to dilute a solution, the number of Dilution Of Solutions In The Lab An example of a dilute solution is. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7. Learn how to dilute and concentrate solutions. A dilution is a solution made by. Dilution Of Solutions In The Lab.
From www.chegg.com
Solved molarity, dilutions, and preparing solutions lab Dilution Of Solutions In The Lab A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Dilution is the addition of solvent,. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7. A dilution is a solution made by adding more solvent to a more concentrated solution. Dilution Of Solutions In The Lab.
From www.shutterstock.com
228 Dilute Solution Concentrated Solution Images, Stock Photos Dilution Of Solutions In The Lab An example of a dilute solution is. Learn how to dilute and concentrate solutions. Dilution is the addition of solvent,. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. A dilution is made by combining a certain volume of reagent or specimen with a certain volume. Dilution Of Solutions In The Lab.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Of Solutions In The Lab An example of a dilute solution is. Understand how stock solutions are used in the laboratory. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. The acid must be diluted. Often, a worker will need to change the concentration of a solution by changing the. Dilution Of Solutions In The Lab.
From saylordotorg.github.io
Solution Concentrations Dilution Of Solutions In The Lab The acid must be diluted. Learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. Dilution is the process of adding more solvent to a solution. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7. An example of a dilute solution is. Serial. Dilution Of Solutions In The Lab.
From www.studypool.com
SOLUTION Standard & Dilution of Solution Studypool Dilution Of Solutions In The Lab Serial dilutions are used in microbiology to reduce bacterial concentrations. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete and masonry surfaces (see figure 14.7.1 14.7. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume. Dilution is the process of weakening or. Dilution Of Solutions In The Lab.
From www.youtube.com
Dilution Lab Results Chemistry Matters YouTube Dilution Of Solutions In The Lab Understand how stock solutions are used in the laboratory. Serial dilutions are used in microbiology to reduce bacterial concentrations. A dilution is a solution made by adding more solvent to a more concentrated solution (stock solution), which reduces the concentration of the solute. A dilution is made by combining a certain volume of reagent or specimen with a certain volume. Dilution Of Solutions In The Lab.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Of Solutions In The Lab The same can be said of a ratio. An example of a dilute solution is. Understand how stock solutions are used in the laboratory. Learn how to dilute and concentrate solutions. Dilution is the process of weakening or deconcentrating a solution. Serial dilutions are used in microbiology to reduce bacterial concentrations. A dilution is made by combining a certain volume. Dilution Of Solutions In The Lab.