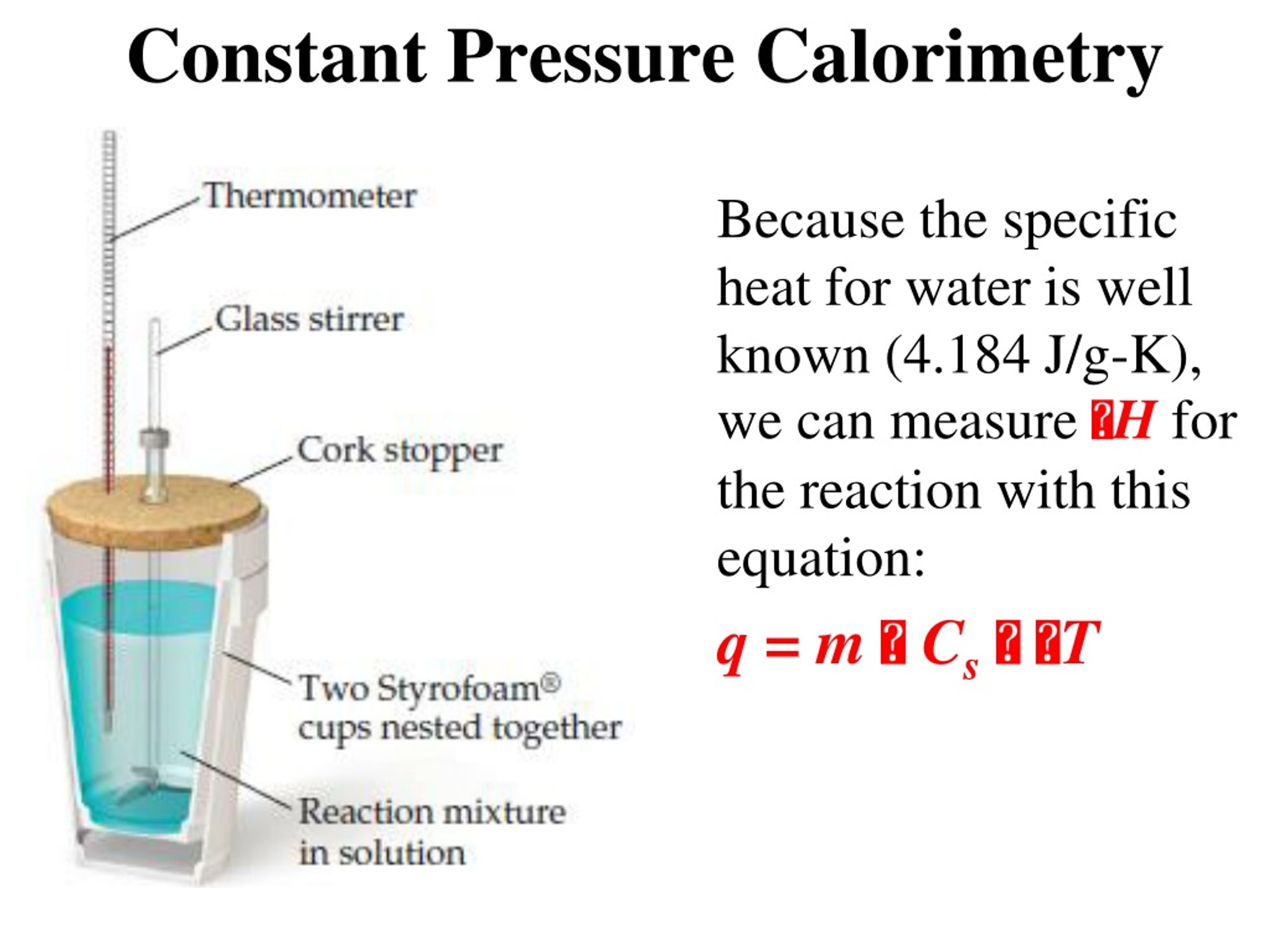
Constant Volume Calorimetry Equation . Calorimetry is used to measure amounts of heat transferred to or from a substance. Constant volume calorimetry formula explained. If the constant volume calorimeter is set up the same way as before, (same steel bomb, same amount of water, etc.) then the heat capacity of. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. W = p δ v and δ v is zero when the volume is constant. Apply the first law of thermodynamics to calorimetry. Δ e = q + w. The fundamental formula for constant volume calorimetry is derived from the first law of. Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. To do so, the heat is exchanged with a calibrated object.
from www.slideserve.com
Constant volume calorimetry formula explained. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. If the constant volume calorimeter is set up the same way as before, (same steel bomb, same amount of water, etc.) then the heat capacity of. The fundamental formula for constant volume calorimetry is derived from the first law of. To do so, the heat is exchanged with a calibrated object. W = p δ v and δ v is zero when the volume is constant. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Calorimetry is used to measure amounts of heat transferred to or from a substance. Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and.
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download
Constant Volume Calorimetry Equation Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. The fundamental formula for constant volume calorimetry is derived from the first law of. To do so, the heat is exchanged with a calibrated object. Apply the first law of thermodynamics to calorimetry. If the constant volume calorimeter is set up the same way as before, (same steel bomb, same amount of water, etc.) then the heat capacity of. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Calorimetry is used to measure amounts of heat transferred to or from a substance. W = p δ v and δ v is zero when the volume is constant. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Constant volume calorimetry formula explained. Δ e = q + w. Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and.
From www.slideserve.com
PPT Zumdahl Chapter 6 PowerPoint Presentation, free download ID4269919 Constant Volume Calorimetry Equation Apply the first law of thermodynamics to calorimetry. The fundamental formula for constant volume calorimetry is derived from the first law of. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. If the constant volume calorimeter is set up the same way as before, (same steel bomb, same amount of water, etc.) then the. Constant Volume Calorimetry Equation.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry Constant Volume Calorimetry Equation Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. W = p δ v and δ v is zero when the volume is constant. If the constant volume calorimeter is set up the same way as before, (same steel bomb, same amount of water, etc.) then the. Constant Volume Calorimetry Equation.
From ar.inspiredpencil.com
Calorimetry Equation Constant Volume Calorimetry Equation So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Constant volume calorimetry formula explained. If the constant volume calorimeter is set up the same way as before, (same steel bomb, same amount of water, etc.) then the heat capacity of. Constant. Constant Volume Calorimetry Equation.
From www.youtube.com
Bomb Calorimetry Example (Constant Volume) YouTube Constant Volume Calorimetry Equation To do so, the heat is exchanged with a calibrated object. Δ e = q + w. Apply the first law of thermodynamics to calorimetry. The fundamental formula for constant volume calorimetry is derived from the first law of. Constant volume calorimetry formula explained. Calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat. Constant Volume Calorimetry Equation.
From www.youtube.com
6.2 part2 AP Chemistry Constant volume calorimetry YouTube Constant Volume Calorimetry Equation W = p δ v and δ v is zero when the volume is constant. Calorimetry is used to measure amounts of heat transferred to or from a substance. Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. If the constant volume calorimeter is set up the. Constant Volume Calorimetry Equation.
From www.slideserve.com
PPT Thermochemistry & Thermodynamics PowerPoint Presentation ID1551469 Constant Volume Calorimetry Equation The fundamental formula for constant volume calorimetry is derived from the first law of. W = p δ v and δ v is zero when the volume is constant. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a. Constant Volume Calorimetry Equation.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube Constant Volume Calorimetry Equation Calorimetry is used to measure amounts of heat transferred to or from a substance. Apply the first law of thermodynamics to calorimetry. The fundamental formula for constant volume calorimetry is derived from the first law of. Constant volume calorimetry formula explained. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Compare heat flow from hot to. Constant Volume Calorimetry Equation.
From ar.inspiredpencil.com
Calorimetry Equation Constant Volume Calorimetry Equation The fundamental formula for constant volume calorimetry is derived from the first law of. Apply the first law of thermodynamics to calorimetry. Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Δ e = q + w. To do so, the heat is exchanged with a calibrated. Constant Volume Calorimetry Equation.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID1551183 Constant Volume Calorimetry Equation So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Δ e = q + w. The fundamental formula for constant volume calorimetry is derived from the first law of. To do so, the heat is exchanged with a calibrated object. Constant volume calorimetry formula explained. Compare heat flow from hot to cold objects in an ideal. Constant Volume Calorimetry Equation.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1875569 Constant Volume Calorimetry Equation So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Constant volume calorimetry formula explained. Apply the first law of thermodynamics to calorimetry. W = p δ v and δ v is zero when the volume is constant. Δ e = q + w. If the constant volume calorimeter is set up the same way as before,. Constant Volume Calorimetry Equation.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID1551148 Constant Volume Calorimetry Equation Apply the first law of thermodynamics to calorimetry. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Constant volume. Constant Volume Calorimetry Equation.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Constant Volume Calorimetry Equation Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. If the constant volume calorimeter is set up the same way as before, (same steel bomb, same amount of water, etc.) then the heat capacity of. Apply the first law of thermodynamics to calorimetry. To do so, the. Constant Volume Calorimetry Equation.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID5405762 Constant Volume Calorimetry Equation So, for a constant volume, δe = q, and therefore, the bomb calorimeter. The fundamental formula for constant volume calorimetry is derived from the first law of. To do so, the heat is exchanged with a calibrated object. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. If the constant volume calorimeter is set. Constant Volume Calorimetry Equation.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Constant Volume Calorimetry Equation To do so, the heat is exchanged with a calibrated object. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Δ e = q + w. The fundamental formula for constant volume calorimetry is. Constant Volume Calorimetry Equation.
From www.pearson.com
Constant Volume Calorimetry Pearson+ Channels Constant Volume Calorimetry Equation Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Δ e = q + w. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding. Constant Volume Calorimetry Equation.
From ar.inspiredpencil.com
Calorimetry Equation Constant Volume Calorimetry Equation Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Constant volume calorimetry formula explained. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Δ e = q +. Constant Volume Calorimetry Equation.
From www.youtube.com
Constantvolume calorimetry Thermodynamics AP Chemistry Khan Constant Volume Calorimetry Equation Apply the first law of thermodynamics to calorimetry. W = p δ v and δ v is zero when the volume is constant. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. To do so, the heat is exchanged with a calibrated object. The fundamental formula for. Constant Volume Calorimetry Equation.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6600582 Constant Volume Calorimetry Equation To do so, the heat is exchanged with a calibrated object. The fundamental formula for constant volume calorimetry is derived from the first law of. Constant volume calorimetry formula explained. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. If the constant volume calorimeter is set up. Constant Volume Calorimetry Equation.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3469636 Constant Volume Calorimetry Equation Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Constant volume calorimetry formula explained. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. If the constant volume calorimeter is set up the same way. Constant Volume Calorimetry Equation.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Constant Volume Calorimetry Equation Constant volume calorimetry formula explained. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. W = p δ v and δ v is zero when the volume is constant. Calorimetry is used to measure amounts of heat transferred to or from a substance. If the constant volume. Constant Volume Calorimetry Equation.
From www.youtube.com
1A 6.5 ConstantVolume Calorimetry YouTube Constant Volume Calorimetry Equation Calorimetry is used to measure amounts of heat transferred to or from a substance. Δ e = q + w. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. To do so, the heat is exchanged with a calibrated object. W = p δ v and. Constant Volume Calorimetry Equation.
From www.w3schools.blog
Cp, Cv calorimetry W3schools Constant Volume Calorimetry Equation So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Constant volume calorimetry formula explained. If the constant volume calorimeter is set up the same way as before, (same steel bomb, same amount of water,. Constant Volume Calorimetry Equation.
From www.slideserve.com
PPT Zumdahl Chapter 6 PowerPoint Presentation, free download ID4269919 Constant Volume Calorimetry Equation Δ e = q + w. Calorimetry is used to measure amounts of heat transferred to or from a substance. Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. The fundamental formula for constant volume calorimetry is derived from the first law of. If the constant volume. Constant Volume Calorimetry Equation.
From slideplayer.com
Thermochemistry Chapter 6 ppt download Constant Volume Calorimetry Equation Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so,. Constant Volume Calorimetry Equation.
From www.chegg.com
Solved 4. In constantvolume calorimetry, the change in Constant Volume Calorimetry Equation Δ e = q + w. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. W = p δ v and δ. Constant Volume Calorimetry Equation.
From www.youtube.com
CHEMISTRY 101 Calculating Change in Internal Energy Using Constant Constant Volume Calorimetry Equation Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Calorimetry is used to measure amounts of heat transferred to or from a substance. Apply the first law of thermodynamics to calorimetry. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. If the constant. Constant Volume Calorimetry Equation.
From slideplayer.com
Enthalpy and Calorimetry ppt download Constant Volume Calorimetry Equation Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Apply the first law of thermodynamics to calorimetry. If the constant volume calorimeter is set up the same way as before, (same steel bomb, same amount of water, etc.) then the heat capacity of. Δ e = q. Constant Volume Calorimetry Equation.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Constant Volume Calorimetry Equation Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Apply the first law of thermodynamics to calorimetry. Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Calorimetry is used to measure amounts of heat. Constant Volume Calorimetry Equation.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Constant Volume Calorimetry Equation Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Apply the first law of thermodynamics to calorimetry. W = p δ v and δ v is zero when the volume is constant. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real.. Constant Volume Calorimetry Equation.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Constant Volume Calorimetry Equation The fundamental formula for constant volume calorimetry is derived from the first law of. If the constant volume calorimeter is set up the same way as before, (same steel bomb, same amount of water, etc.) then the heat capacity of. Δ e = q + w. Apply the first law of thermodynamics to calorimetry. Constant volume calorimetry, also known as. Constant Volume Calorimetry Equation.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry Constant Volume Calorimetry Equation If the constant volume calorimeter is set up the same way as before, (same steel bomb, same amount of water, etc.) then the heat capacity of. To do so, the heat is exchanged with a calibrated object. Apply the first law of thermodynamics to calorimetry. Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of. Constant Volume Calorimetry Equation.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Constant Volume Calorimetry Equation Apply the first law of thermodynamics to calorimetry. If the constant volume calorimeter is set up the same way as before, (same steel bomb, same amount of water, etc.) then the heat capacity of. Constant volume calorimetry formula explained. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. W = p δ v and δ v. Constant Volume Calorimetry Equation.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID5405762 Constant Volume Calorimetry Equation The fundamental formula for constant volume calorimetry is derived from the first law of. Constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. W = p δ v and δ v is zero when the volume is constant. To do so, the heat is exchanged with a. Constant Volume Calorimetry Equation.
From www.youtube.com
ConstantVolume Calorimetry YouTube Constant Volume Calorimetry Equation If the constant volume calorimeter is set up the same way as before, (same steel bomb, same amount of water, etc.) then the heat capacity of. Apply the first law of thermodynamics to calorimetry. W = p δ v and δ v is zero when the volume is constant. Compare heat flow from hot to cold objects in an ideal. Constant Volume Calorimetry Equation.
From www.scribd.com
Measuring E For Chemical Reactions ConstantVolume Calorimetry PDF Constant Volume Calorimetry Equation Constant volume calorimetry formula explained. To do so, the heat is exchanged with a calibrated object. W = p δ v and δ v is zero when the volume is constant. So, for a constant volume, δe = q, and therefore, the bomb calorimeter. Apply the first law of thermodynamics to calorimetry. Constant volume calorimetry, also know as bomb calorimetry,. Constant Volume Calorimetry Equation.