Enzyme Catalysis Example In Chemistry . enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. definition of an enzyme. Homolytic vs heterolytic bond cleavage. Activation energy and the reaction coordinate. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of.
from www.chegg.com
enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. definition of an enzyme. enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. Activation energy and the reaction coordinate. Homolytic vs heterolytic bond cleavage. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme.
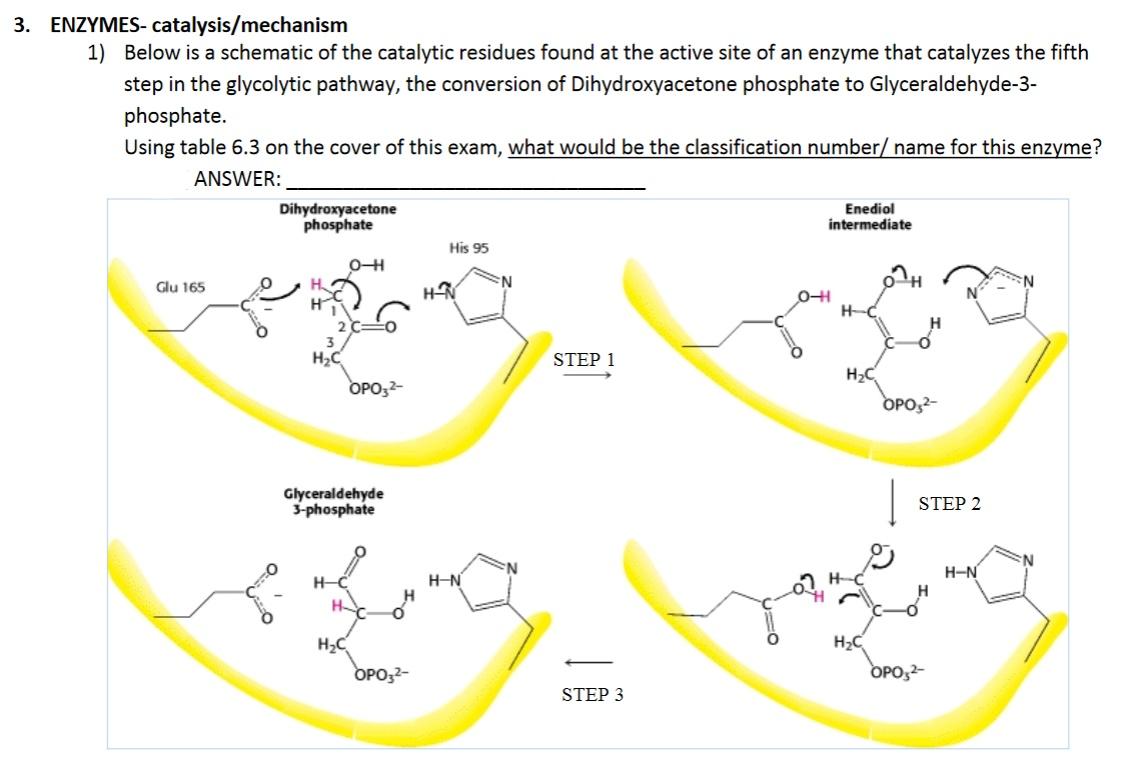
Solved 3. ENZYMES catalysis/mechanism 1) Below is a
Enzyme Catalysis Example In Chemistry In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. Activation energy and the reaction coordinate. definition of an enzyme. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. Homolytic vs heterolytic bond cleavage.
From webapi.bu.edu
⛔ Acid base enzyme catalysis. Enzyme Catalysis. 20221026 Enzyme Catalysis Example In Chemistry definition of an enzyme. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. Homolytic vs heterolytic bond cleavage. Activation energy and the reaction coordinate. enzymes are a. Enzyme Catalysis Example In Chemistry.
From www.slideserve.com
PPT ENZYME CATALYSIS PowerPoint Presentation, free download ID4176592 Enzyme Catalysis Example In Chemistry enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature. Enzyme Catalysis Example In Chemistry.
From slidetodoc.com
ENZYME BIOLOGICAL CATALYST Enzyme As Catalyst All enzymes Enzyme Catalysis Example In Chemistry Homolytic vs heterolytic bond cleavage. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. definition of an enzyme. Activation. Enzyme Catalysis Example In Chemistry.
From fity.club
Enzyme The Catalyst Enzyme Catalysis Example In Chemistry enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of.. Enzyme Catalysis Example In Chemistry.
From www.slideserve.com
PPT Outline of Enzymes PowerPoint Presentation ID364722 Enzyme Catalysis Example In Chemistry In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. Activation energy and the reaction coordinate. definition of an enzyme.. Enzyme Catalysis Example In Chemistry.
From www.chegg.com
Solved 3. ENZYMES catalysis/mechanism 1) Below is a Enzyme Catalysis Example In Chemistry In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. Activation energy and the reaction coordinate. enzymes are a class of catalysts that are responsible for. Enzyme Catalysis Example In Chemistry.
From www.pinterest.co.uk
Catalyst and Mechanism of Enzyme Catalysis Enzymes, Chemistry, Active Enzyme Catalysis Example In Chemistry enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. Homolytic vs heterolytic bond cleavage. Activation energy and the reaction coordinate. enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. for example, the enzyme carbonic anhydrase accelerates the above reaction at. Enzyme Catalysis Example In Chemistry.
From www.slideserve.com
PPT 23.5 Features of homogeneous catalysis PowerPoint Presentation Enzyme Catalysis Example In Chemistry enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. definition of an enzyme. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. Homolytic vs heterolytic bond cleavage. Activation energy and the reaction coordinate. . Enzyme Catalysis Example In Chemistry.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of Enzyme Catalysis Example In Chemistry In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. Homolytic vs heterolytic bond cleavage. Activation energy and the reaction coordinate.. Enzyme Catalysis Example In Chemistry.
From zymvol.com
All you need to know about enzymes Zymvol Enzyme Catalysis Example In Chemistry definition of an enzyme. enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. Homolytic vs heterolytic bond cleavage. Activation energy and the reaction coordinate. . Enzyme Catalysis Example In Chemistry.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Enzyme Catalysis Example In Chemistry enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. Homolytic vs heterolytic bond cleavage. for example, the enzyme carbonic anhydrase accelerates the above reaction at. Enzyme Catalysis Example In Chemistry.
From biology4ibdp.weebly.com
2.5 Enzymes BIOLOGY4IBDP Enzyme Catalysis Example In Chemistry Homolytic vs heterolytic bond cleavage. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. In the first step, an enzyme molecule (e) and the substrate molecule. Enzyme Catalysis Example In Chemistry.
From www.slideserve.com
PPT ENZYME CATALYSIS PowerPoint Presentation, free download ID4176592 Enzyme Catalysis Example In Chemistry for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. enzymes are a class of catalysts that are responsible for. Enzyme Catalysis Example In Chemistry.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation, free download ID3496562 Enzyme Catalysis Example In Chemistry Activation energy and the reaction coordinate. enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. Homolytic vs heterolytic bond cleavage. In the first step, an enzyme molecule (e) and the substrate molecule. Enzyme Catalysis Example In Chemistry.
From www.slideserve.com
PPT CHMI 2227E Biochemistry I PowerPoint Presentation, free download Enzyme Catalysis Example In Chemistry Homolytic vs heterolytic bond cleavage. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. definition of an enzyme. Activation energy and the reaction coordinate. enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many. Enzyme Catalysis Example In Chemistry.
From www.slideserve.com
PPT CHMI 2227E Biochemistry I PowerPoint Presentation, free download Enzyme Catalysis Example In Chemistry Activation energy and the reaction coordinate. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. Homolytic vs heterolytic bond cleavage. definition of an enzyme. . Enzyme Catalysis Example In Chemistry.
From www.dreamstime.com
Substrate and Enzyme in Catalytic Cycle Stock Vector Illustration of Enzyme Catalysis Example In Chemistry Activation energy and the reaction coordinate. enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. definition of an enzyme. In the first step, an enzyme. Enzyme Catalysis Example In Chemistry.
From biologyease.com
Mechanism of Enzyme Catalysis Biology Ease Enzyme Catalysis Example In Chemistry Homolytic vs heterolytic bond cleavage. definition of an enzyme. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. Activation energy and the reaction coordinate. In the first step,. Enzyme Catalysis Example In Chemistry.
From biology.stackexchange.com
cell biology What advantage does an enzyme serve over catalysis by Enzyme Catalysis Example In Chemistry enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. Activation energy and the reaction coordinate. Homolytic vs heterolytic bond cleavage. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. for example, the enzyme carbonic. Enzyme Catalysis Example In Chemistry.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Enzyme Catalysis Example In Chemistry enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. Homolytic vs heterolytic bond cleavage. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. Activation energy and the reaction coordinate. definition of. Enzyme Catalysis Example In Chemistry.
From www.slideserve.com
PPT ENZYME BIOLOGICAL CATALYST PowerPoint Presentation, free Enzyme Catalysis Example In Chemistry enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of. Enzyme Catalysis Example In Chemistry.
From slidetodoc.com
LECTURE 2 ENZYME GENERAL PRINCIPLES OF CATALYSIS Enzyme Catalysis Example In Chemistry enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms.. Enzyme Catalysis Example In Chemistry.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Enzyme Catalysis Example In Chemistry enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. Homolytic vs heterolytic bond cleavage. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. definition of an enzyme. enzymes are a class of catalysts. Enzyme Catalysis Example In Chemistry.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Enzyme Catalysis Example In Chemistry for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. Activation energy and the reaction coordinate. definition of an enzyme. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme.. Enzyme Catalysis Example In Chemistry.
From www.youtube.com
Characteristics of Enzyme Catalysis Part 11 Cbse grade surface 12 Enzyme Catalysis Example In Chemistry definition of an enzyme. Activation energy and the reaction coordinate. enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. In the first step, an enzyme molecule (e) and the substrate molecule. Enzyme Catalysis Example In Chemistry.
From www.thoughtco.com
Structure and Function of an Enzyme Enzyme Catalysis Example In Chemistry In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. enzymes are a class of catalysts that are responsible for. Enzyme Catalysis Example In Chemistry.
From www.pinterest.com
Image from Enzyme Catalysis Example In Chemistry definition of an enzyme. enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. Homolytic vs heterolytic bond cleavage. enzymes are a class of catalysts that are responsible. Enzyme Catalysis Example In Chemistry.
From infinitabiotech.com
Characteristics Of Enzyme Catalysis Infinita Biotech Enzyme Catalysis Example In Chemistry definition of an enzyme. Activation energy and the reaction coordinate. enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. Homolytic vs heterolytic. Enzyme Catalysis Example In Chemistry.
From www.slideserve.com
PPT ENZYME BIOLOGICAL CATALYST PowerPoint Presentation, free Enzyme Catalysis Example In Chemistry definition of an enzyme. Homolytic vs heterolytic bond cleavage. enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. Activation energy and the reaction coordinate. In the first step, an enzyme molecule. Enzyme Catalysis Example In Chemistry.
From www.youtube.com
01.10 Base Catalysis and Summary YouTube Enzyme Catalysis Example In Chemistry definition of an enzyme. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. for example, the enzyme carbonic anhydrase accelerates the. Enzyme Catalysis Example In Chemistry.
From gwendolynkruwmejia.blogspot.com
Whay Analogy Is Used to Describe Enzyme Substrate Interaction Enzyme Catalysis Example In Chemistry enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms.. Enzyme Catalysis Example In Chemistry.
From slidetodoc.com
ENZYME BIOLOGICAL CATALYST Enzyme As Catalyst All enzymes Enzyme Catalysis Example In Chemistry Activation energy and the reaction coordinate. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and react to form an intermediate compound called the enzyme. enzyme catalysts are complex nitrogenous proteins that help to catalyse the biochemical reactions in living organisms. for example, the enzyme carbonic anhydrase accelerates the above reaction. Enzyme Catalysis Example In Chemistry.
From www.slideserve.com
PPT Chymosin Lab PowerPoint Presentation, free download ID484941 Enzyme Catalysis Example In Chemistry enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. definition of an enzyme. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. In the first step, an enzyme molecule (e) and the substrate molecule. Enzyme Catalysis Example In Chemistry.
From ibiologia.com
Enzymes Definition, Classification & Functions Enzyme Catalysis Example In Chemistry Activation energy and the reaction coordinate. enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. Homolytic vs heterolytic bond cleavage. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. enzyme catalysts are complex nitrogenous. Enzyme Catalysis Example In Chemistry.
From jadagrocantu.blogspot.com
Explain the Difference Between a Catalyst and an Enzyme Enzyme Catalysis Example In Chemistry Activation energy and the reaction coordinate. Homolytic vs heterolytic bond cleavage. for example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times the rate of. enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical. definition of an enzyme. In. Enzyme Catalysis Example In Chemistry.