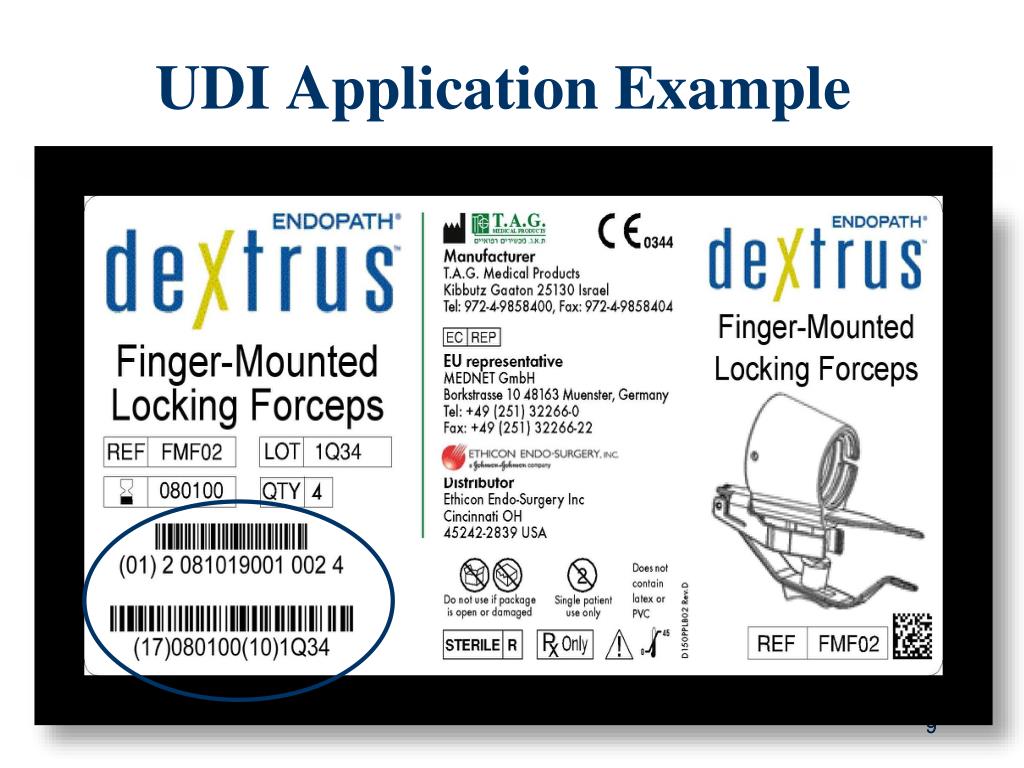
Udi Medical Device Example . To ensure a globally standardized and harmonized system, the. accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. Us food and drug administration website,. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. the european union database on medical device (eudamed) is a digital platform for legal information on medical. according to the u.s. Put the device identifier (di) on the device label in. a udi code aims at unambiguous identification of a specific medical device. on the surface, unique device identification (udi) is a simple concept.
from www.slideserve.com
accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. To ensure a globally standardized and harmonized system, the. a udi code aims at unambiguous identification of a specific medical device. Put the device identifier (di) on the device label in. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. Us food and drug administration website,. on the surface, unique device identification (udi) is a simple concept. according to the u.s. the european union database on medical device (eudamed) is a digital platform for legal information on medical.
PPT Unique Device Identification (UDI) Enabling the Transformation
Udi Medical Device Example on the surface, unique device identification (udi) is a simple concept. Put the device identifier (di) on the device label in. the european union database on medical device (eudamed) is a digital platform for legal information on medical. To ensure a globally standardized and harmonized system, the. according to the u.s. Us food and drug administration website,. on the surface, unique device identification (udi) is a simple concept. a udi code aims at unambiguous identification of a specific medical device. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Udi Medical Device Example Us food and drug administration website,. accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. the european union database on medical device (eudamed) is a digital platform for legal. Udi Medical Device Example.
From medicaldevicehq.com
UDI requirements for medical device manufacturers in the EU Medical Udi Medical Device Example Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. To ensure a globally standardized and harmonized system, the. on the surface, unique device identification (udi) is a simple concept. accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,.. Udi Medical Device Example.
From easymedicaldevice.com
UDI Beginners Guide Unique Device Identification (EU MDR and IVDR) Udi Medical Device Example Put the device identifier (di) on the device label in. on the surface, unique device identification (udi) is a simple concept. according to the u.s. the european union database on medical device (eudamed) is a digital platform for legal information on medical. Fda, when fully implemented, the label of most devices will include a unique device identifier. Udi Medical Device Example.
From www.slideserve.com
PPT Unique Device Identification (UDI) Enabling the Transformation Udi Medical Device Example a udi code aims at unambiguous identification of a specific medical device. Us food and drug administration website,. the european union database on medical device (eudamed) is a digital platform for legal information on medical. To ensure a globally standardized and harmonized system, the. accurate, comprehensive product information gleaned from udids is essential to ensure the correct. Udi Medical Device Example.
From www.slideserve.com
PPT Unique Device Identification (UDI) Enabling the Transformation Udi Medical Device Example accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. according to the u.s. the european union database on medical device (eudamed) is a digital platform for legal information on medical. Us food and drug administration website,. To ensure a globally standardized and harmonized system, the.. Udi Medical Device Example.
From www.greenlight.guru
Ultimate Guide to UDI for Medical Devices Udi Medical Device Example according to the u.s. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. on the surface, unique device identification (udi) is a simple concept. To ensure a globally standardized and harmonized system, the. Put the device identifier (di) on the device label in. accurate, comprehensive product information gleaned from. Udi Medical Device Example.
From barcode-labels.com
Medical Devices Electronic Imaging Materials Udi Medical Device Example a udi code aims at unambiguous identification of a specific medical device. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. the european union database on medical device (eudamed) is a digital platform for legal information on medical. Put the device identifier (di) on the device label in. on. Udi Medical Device Example.
From www.celegence.com
Medical Device UDI Requirements in the US and Europe Udi Medical Device Example the european union database on medical device (eudamed) is a digital platform for legal information on medical. accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. Us food and drug administration website,. Fda, when fully implemented, the label of most devices will include a unique device. Udi Medical Device Example.
From easymedicaldevice.com
UDI Beginners Guide Unique Device Identification (EU MDR and IVDR) Udi Medical Device Example accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. a udi code aims at unambiguous identification of a specific medical device. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. Us food and drug administration website,. on. Udi Medical Device Example.
From www.slideserve.com
PPT Unique Device Identification (UDI) Transforming the Global Udi Medical Device Example according to the u.s. a udi code aims at unambiguous identification of a specific medical device. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. on the surface, unique device identification (udi) is a simple concept. the european union database on medical device (eudamed) is a digital platform. Udi Medical Device Example.
From www.nicelabel.com
FDA UDI compliant labelling NiceLabel Udi Medical Device Example a udi code aims at unambiguous identification of a specific medical device. accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. To ensure a globally standardized and harmonized system, the. Us food and drug administration website,. on the surface, unique device identification (udi) is a. Udi Medical Device Example.
From easymedicaldevice.com
UDI Beginners Guide Unique Device Identification (EU MDR and IVDR) Udi Medical Device Example Put the device identifier (di) on the device label in. on the surface, unique device identification (udi) is a simple concept. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. a udi code aims at unambiguous identification of a specific medical device. accurate, comprehensive product information gleaned from udids. Udi Medical Device Example.
From operonstrategist.com
UDIUnique Device Identification System (Easily Comply Your Medical Udi Medical Device Example Us food and drug administration website,. accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. Put the device identifier (di) on the device label in. a udi code aims at unambiguous identification of a specific medical device. on the surface, unique device identification (udi) is. Udi Medical Device Example.
From pkgcompliance.com
Take Aim. It's UDI Season. Packaging Compliance Labs Udi Medical Device Example the european union database on medical device (eudamed) is a digital platform for legal information on medical. To ensure a globally standardized and harmonized system, the. Put the device identifier (di) on the device label in. accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. . Udi Medical Device Example.
From www.reedtech.com
Unique Device Identification (UDI) Lexis Nexis Reed Tech Udi Medical Device Example To ensure a globally standardized and harmonized system, the. on the surface, unique device identification (udi) is a simple concept. Us food and drug administration website,. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. Put the device identifier (di) on the device label in. a udi code aims at. Udi Medical Device Example.
From www.greenlight.guru
Ultimate Guide to UDI for Medical Devices Udi Medical Device Example the european union database on medical device (eudamed) is a digital platform for legal information on medical. Put the device identifier (di) on the device label in. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. on the surface, unique device identification (udi) is a simple concept. a udi. Udi Medical Device Example.
From paragondsi.com
UDI Unique Device Identification for Single and Multiple Uses Udi Medical Device Example Us food and drug administration website,. the european union database on medical device (eudamed) is a digital platform for legal information on medical. Put the device identifier (di) on the device label in. To ensure a globally standardized and harmonized system, the. according to the u.s. Fda, when fully implemented, the label of most devices will include a. Udi Medical Device Example.
From easymedicaldevice.com
UDI Beginners Guide Unique Device Identification (EU MDR and IVDR) Udi Medical Device Example accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. the european union database on medical device (eudamed) is a digital platform for legal information on medical. Us food and drug administration website,. Fda, when fully implemented, the label of most devices will include a unique device. Udi Medical Device Example.
From easymedicaldevice.com
UDI Beginners Guide Unique Device Identification (EU MDR and IVDR) Udi Medical Device Example Us food and drug administration website,. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. Put the device identifier (di) on the device label in. a udi code aims at unambiguous identification of a specific medical device. on the surface, unique device identification (udi) is a simple concept. according. Udi Medical Device Example.
From bzt-ar.com
UDI BZTAR Udi Medical Device Example Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. according to the u.s. the european union database on medical device (eudamed) is a digital platform for legal information on medical. a udi code aims at unambiguous identification of a specific medical device. accurate, comprehensive product information gleaned from. Udi Medical Device Example.
From www.slideserve.com
PPT Unique Device Identification (UDI) Transforming the Global Udi Medical Device Example To ensure a globally standardized and harmonized system, the. Put the device identifier (di) on the device label in. on the surface, unique device identification (udi) is a simple concept. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. a udi code aims at unambiguous identification of a specific medical. Udi Medical Device Example.
From www.barcode-us.com
Medical Devices UDI Udi Medical Device Example the european union database on medical device (eudamed) is a digital platform for legal information on medical. Us food and drug administration website,. Put the device identifier (di) on the device label in. accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. a udi code. Udi Medical Device Example.
From easymedicaldevice.com
UDI Beginners Guide Unique Device Identification (EU MDR and IVDR) Udi Medical Device Example according to the u.s. a udi code aims at unambiguous identification of a specific medical device. accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. Us food and drug administration website,. Fda, when fully implemented, the label of most devices will include a unique device. Udi Medical Device Example.
From www.greenlight.guru
Ultimate Guide to UDI for Medical Devices Udi Medical Device Example according to the u.s. Put the device identifier (di) on the device label in. on the surface, unique device identification (udi) is a simple concept. a udi code aims at unambiguous identification of a specific medical device. To ensure a globally standardized and harmonized system, the. accurate, comprehensive product information gleaned from udids is essential to. Udi Medical Device Example.
From abr.com
Label Compliance AB&R® (American Barcode and RFID) Udi Medical Device Example accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. according to the u.s. on the surface, unique device identification (udi) is a simple concept. Put the device identifier (di) on the device label in. To ensure a globally standardized and harmonized system, the. Us food. Udi Medical Device Example.
From www.youtube.com
FDA UDI Regulation’s Impact on Medical Device Labelers inar YouTube Udi Medical Device Example the european union database on medical device (eudamed) is a digital platform for legal information on medical. accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. according to the u.s. Us food and drug administration website,. To ensure a globally standardized and harmonized system, the.. Udi Medical Device Example.
From www.greenlight.guru
Ultimate Guide to UDI for Medical Devices Udi Medical Device Example on the surface, unique device identification (udi) is a simple concept. a udi code aims at unambiguous identification of a specific medical device. the european union database on medical device (eudamed) is a digital platform for legal information on medical. Us food and drug administration website,. To ensure a globally standardized and harmonized system, the. Put the. Udi Medical Device Example.
From www.slideshare.net
FDA Unique Device Identification (UDI) Overview Udi Medical Device Example Put the device identifier (di) on the device label in. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. a udi code aims at unambiguous identification of a specific medical device. on the surface, unique device identification (udi) is a simple concept. the european union database on medical device. Udi Medical Device Example.
From medicaldevicehq.com
UDI requirements for medical device manufacturers in the EU Medical Udi Medical Device Example the european union database on medical device (eudamed) is a digital platform for legal information on medical. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. Us food and drug administration website,. accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices. Udi Medical Device Example.
From www.reedtech.com
UDI A Guide to Device Identifiers Lexis Nexis Reed Tech Udi Medical Device Example on the surface, unique device identification (udi) is a simple concept. Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. the european union database on medical device (eudamed). Udi Medical Device Example.
From easymedicaldevice.com
UDI Beginners Guide Unique Device Identification (EU MDR and IVDR) Udi Medical Device Example Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. Us food and drug administration website,. the european union database on medical device (eudamed) is a digital platform for legal information on medical. a udi code aims at unambiguous identification of a specific medical device. Put the device identifier (di) on. Udi Medical Device Example.
From www.slideserve.com
PPT Unique Device Identification (UDI) Enabling the Transformation Udi Medical Device Example To ensure a globally standardized and harmonized system, the. the european union database on medical device (eudamed) is a digital platform for legal information on medical. on the surface, unique device identification (udi) is a simple concept. Put the device identifier (di) on the device label in. according to the u.s. Us food and drug administration website,.. Udi Medical Device Example.
From medicaldevicehq.com
UDI requirements for medical device manufacturers in the EU Medical Udi Medical Device Example Fda, when fully implemented, the label of most devices will include a unique device identifier (udi) in. on the surface, unique device identification (udi) is a simple concept. Put the device identifier (di) on the device label in. a udi code aims at unambiguous identification of a specific medical device. To ensure a globally standardized and harmonized system,. Udi Medical Device Example.
From www.advena.mt
EU UDI Requirements, Definition, and Guidance Unique Device Identifier Udi Medical Device Example according to the u.s. a udi code aims at unambiguous identification of a specific medical device. on the surface, unique device identification (udi) is a simple concept. accurate, comprehensive product information gleaned from udids is essential to ensure the correct and safe use of devices by all users,. Us food and drug administration website,. To ensure. Udi Medical Device Example.
From www.gs1.ch
UDI Unique Device Identification GS1 Switzerland Udi Medical Device Example the european union database on medical device (eudamed) is a digital platform for legal information on medical. Us food and drug administration website,. Put the device identifier (di) on the device label in. according to the u.s. on the surface, unique device identification (udi) is a simple concept. accurate, comprehensive product information gleaned from udids is. Udi Medical Device Example.