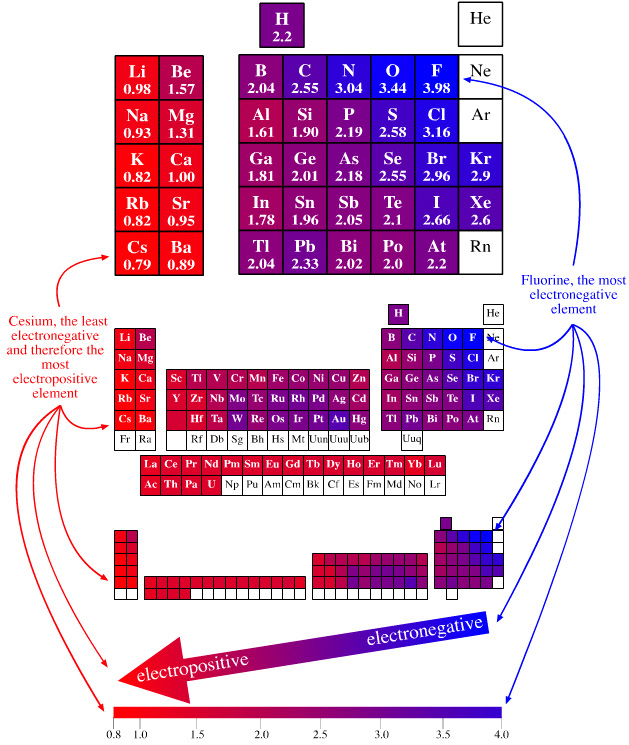
Hydrogen Chlorine Electronegativity . learn how electronegativity measures the tendency of an atom to attract a bonding pair of electrons. Find out the electronegativity of. calculate the type of bond between elements based on their electronegativity values using this tool. learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. why does electronegativity fall as you go down a group? the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines the polarity of covalent bonds. Think of hydrogen fluoride and hydrogen chloride. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound.
from exoyzbldb.blob.core.windows.net
calculate the type of bond between elements based on their electronegativity values using this tool. the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. Think of hydrogen fluoride and hydrogen chloride. Find out the electronegativity of. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. learn how electronegativity measures the tendency of an atom to attract a bonding pair of electrons. learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines the polarity of covalent bonds. why does electronegativity fall as you go down a group?
Is Chlorine Electronegative Or Electropositive at Otis King blog
Hydrogen Chlorine Electronegativity learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. Find out the electronegativity of. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. why does electronegativity fall as you go down a group? learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines the polarity of covalent bonds. Think of hydrogen fluoride and hydrogen chloride. calculate the type of bond between elements based on their electronegativity values using this tool. learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. learn how electronegativity measures the tendency of an atom to attract a bonding pair of electrons.
From www.britannica.com
Chemical compound Trends in the chemical properties of the elements Britannica Hydrogen Chlorine Electronegativity calculate the type of bond between elements based on their electronegativity values using this tool. Find out the electronegativity of. learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines the polarity of covalent bonds.. Hydrogen Chlorine Electronegativity.
From www.numerade.com
SOLVED For the compounds HCI and SO2, with the element information given in the table, Element Hydrogen Chlorine Electronegativity 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. Think of hydrogen fluoride and hydrogen chloride. learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines the. Hydrogen Chlorine Electronegativity.
From www.bigstockphoto.com
Electronegativity Image & Photo (Free Trial) Bigstock Hydrogen Chlorine Electronegativity why does electronegativity fall as you go down a group? Find out the electronegativity of. Think of hydrogen fluoride and hydrogen chloride. learn how electronegativity measures the tendency of an atom to attract a bonding pair of electrons. calculate the type of bond between elements based on their electronegativity values using this tool. learn how electronegativity. Hydrogen Chlorine Electronegativity.
From exocoyzqy.blob.core.windows.net
Does Chlorine Have A High Electronegativity at Gordon Maxwell blog Hydrogen Chlorine Electronegativity the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. Find out the electronegativity of. why does electronegativity fall as you go down a group? learn how electronegativity measures the tendency of an atom to attract a bonding. Hydrogen Chlorine Electronegativity.
From ar.inspiredpencil.com
Electronegativity Values Hydrogen Chlorine Electronegativity learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. learn how electronegativity measures the tendency of an atom to attract a bonding pair of electrons. why does electronegativity fall as you go down a group? calculate the type of bond between elements based on their electronegativity. Hydrogen Chlorine Electronegativity.
From www.slideserve.com
PPT Electronegativity PowerPoint Presentation, free download ID256407 Hydrogen Chlorine Electronegativity Think of hydrogen fluoride and hydrogen chloride. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. Find out the electronegativity of. learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines the polarity of covalent bonds. calculate the type of. Hydrogen Chlorine Electronegativity.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Hydrogen Chlorine Electronegativity the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. Think of hydrogen fluoride and hydrogen chloride. learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. learn how to define. Hydrogen Chlorine Electronegativity.
From www.dreamstime.com
Chlorine Chemical Element with First Ionization Energy, Atomic Mass and Electronegativity Values Hydrogen Chlorine Electronegativity the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. Think of hydrogen fluoride and hydrogen chloride. calculate the type of bond between elements based on their electronegativity values using this tool. Find out the electronegativity of. learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines. Hydrogen Chlorine Electronegativity.
From iperiodictable.com
What is Electronegativity Chart List of Electronegativity [PDF] Periodic Table Hydrogen Chlorine Electronegativity the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. learn how electronegativity measures the tendency of an atom to attract a bonding pair of electrons. learn how electronegativity measures the tendency of an atom to attract electrons. Hydrogen Chlorine Electronegativity.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Hydrogen Chlorine Electronegativity calculate the type of bond between elements based on their electronegativity values using this tool. the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. why does electronegativity fall as you go down a group? learn how electronegativity measures the tendency of an atom to attract a bonding pair of electrons. learn. Hydrogen Chlorine Electronegativity.
From www.vedantu.com
With respect of chlorine, hydrogen will be(A) Electropositive(B) Electronegative(C) Neutral(D Hydrogen Chlorine Electronegativity Find out the electronegativity of. learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. . Hydrogen Chlorine Electronegativity.
From pdfslide.net
Electronegativity + 0 0 HClHH The basic units ionic vs. covalent Ionic compounds form Hydrogen Chlorine Electronegativity learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. Find out the electronegativity of. learn how to define and measure. Hydrogen Chlorine Electronegativity.
From www.numerade.com
SOLVED PLEASE HELP 15 POINTS AND MARKED BRAINLIST... Use the chart to determine which pair of Hydrogen Chlorine Electronegativity Find out the electronegativity of. Think of hydrogen fluoride and hydrogen chloride. learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. learn how electronegativity measures the tendency of an atom to attract a bonding pair of electrons. why does electronegativity fall as you go down a group? 119 rows find. Hydrogen Chlorine Electronegativity.
From www.nagwa.com
Question Video Understanding How Electron Density Is Distributed in Hydrogen Chloride Molecules Hydrogen Chlorine Electronegativity Think of hydrogen fluoride and hydrogen chloride. why does electronegativity fall as you go down a group? learn how electronegativity measures the tendency of an atom to attract a bonding pair of electrons. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. learn how electronegativity measures the tendency. Hydrogen Chlorine Electronegativity.
From exozjrgwj.blob.core.windows.net
Lewis Dot Structure For Chlorine Atom at Ernest Silverstein blog Hydrogen Chlorine Electronegativity the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. Think of hydrogen fluoride and hydrogen chloride. why does electronegativity fall as you go down a group? Find out the electronegativity of. learn how electronegativity determines. Hydrogen Chlorine Electronegativity.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Hydrogen Chlorine Electronegativity why does electronegativity fall as you go down a group? learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. calculate the type of bond between elements based on their electronegativity values using this tool. learn how electronegativity measures the tendency of an atom to attract electrons. Hydrogen Chlorine Electronegativity.
From lessoncampuspolypide.z5.web.core.windows.net
Electronegativity And Polarity Chart Hydrogen Chlorine Electronegativity calculate the type of bond between elements based on their electronegativity values using this tool. why does electronegativity fall as you go down a group? Find out the electronegativity of. the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. Think of hydrogen fluoride and hydrogen chloride. 119 rows find the electronegativity values. Hydrogen Chlorine Electronegativity.
From www.science-revision.co.uk
Electronegativity and polar bonds Hydrogen Chlorine Electronegativity Think of hydrogen fluoride and hydrogen chloride. calculate the type of bond between elements based on their electronegativity values using this tool. learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines the polarity of covalent bonds. the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44.. Hydrogen Chlorine Electronegativity.
From es.dreamstime.com
Tabla Periódica Del Electronegativity Imagen de archivo Imagen 38153931 Hydrogen Chlorine Electronegativity calculate the type of bond between elements based on their electronegativity values using this tool. why does electronegativity fall as you go down a group? Think of hydrogen fluoride and hydrogen chloride. learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines the polarity of covalent bonds. 119 rows. Hydrogen Chlorine Electronegativity.
From exoyzbldb.blob.core.windows.net
Is Chlorine Electronegative Or Electropositive at Otis King blog Hydrogen Chlorine Electronegativity learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines the polarity of covalent bonds. learn how electronegativity measures the tendency of an atom to attract a bonding pair of electrons. Think of hydrogen fluoride and hydrogen chloride. 119 rows find the electronegativity values of all the elements in a. Hydrogen Chlorine Electronegativity.
From www.youtube.com
Electronegativity, Basic Introduction, Periodic Trends Which Element Is More Electronegative Hydrogen Chlorine Electronegativity learn how electronegativity measures the tendency of an atom to attract a bonding pair of electrons. calculate the type of bond between elements based on their electronegativity values using this tool. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. learn how to define and measure electronegativity, the. Hydrogen Chlorine Electronegativity.
From www.youtube.com
Covalent Bond of Hydrogen , Chlorine and Hydro Chloride Molecule (Different Kind Of Bonds Hydrogen Chlorine Electronegativity the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines the polarity of covalent bonds. why does electronegativity fall as you go down a group? learn how to define and measure electronegativity, the ability of an. Hydrogen Chlorine Electronegativity.
From www.slideshare.net
Electronegativity part two Hydrogen Chlorine Electronegativity why does electronegativity fall as you go down a group? calculate the type of bond between elements based on their electronegativity values using this tool. Think of hydrogen fluoride and hydrogen chloride. learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. learn how electronegativity measures the tendency of an atom. Hydrogen Chlorine Electronegativity.
From slideplayer.com
State University of New York at Brockport ppt download Hydrogen Chlorine Electronegativity learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines the polarity of covalent bonds. Think of hydrogen fluoride and hydrogen chloride. learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. calculate the type of bond between elements based on their electronegativity values using. Hydrogen Chlorine Electronegativity.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Hydrogen Chlorine Electronegativity Think of hydrogen fluoride and hydrogen chloride. the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. Find out the electronegativity of. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. calculate the type of bond between elements based on their electronegativity values using. Hydrogen Chlorine Electronegativity.
From chem.libretexts.org
8.7 Bond Polarity and Electronegativity Chemistry LibreTexts Hydrogen Chlorine Electronegativity learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. Find out the electronegativity of. Think. Hydrogen Chlorine Electronegativity.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Hydrogen Chlorine Electronegativity Think of hydrogen fluoride and hydrogen chloride. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. learn how electronegativity measures the tendency of an atom to attract a bonding pair of electrons. learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic.. Hydrogen Chlorine Electronegativity.
From slideplayer.com
Chapter 8 Covalent Bonding 8.4 Polar Bonds and Molecules ppt download Hydrogen Chlorine Electronegativity learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical compound. calculate the type of bond between elements based on their electronegativity values using this tool. why does electronegativity fall as you go down a group? learn how electronegativity measures the tendency of an atom to attract a. Hydrogen Chlorine Electronegativity.
From www.numerade.com
SOLVED Sodium (Na) and hydrogen (H) have the same number of electrons in their outer shells but Hydrogen Chlorine Electronegativity learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines the polarity of covalent bonds. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. Think of hydrogen fluoride and hydrogen chloride. Find out the electronegativity of. learn how electronegativity determines the type. Hydrogen Chlorine Electronegativity.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Chlorine Hydrogen Chlorine Electronegativity Find out the electronegativity of. the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. learn how electronegativity measures the tendency. Hydrogen Chlorine Electronegativity.
From www.slideshare.net
Covalent Bonding Chapter 8 Hydrogen Chlorine Electronegativity learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines the polarity of covalent bonds. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. learn how to define and measure electronegativity, the ability of an atom to attract electrons in a chemical. Hydrogen Chlorine Electronegativity.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Hydrogen Chlorine Electronegativity learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines the polarity of covalent bonds. learn how electronegativity measures the tendency of an atom to attract a bonding pair of electrons. why does electronegativity fall as you go down a group? the electronegativity of hydrogen (h) is 2.18 and. Hydrogen Chlorine Electronegativity.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Hydrogen Chlorine Electronegativity learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. Think of hydrogen fluoride and hydrogen chloride. learn how electronegativity measures the tendency of an atom to attract a bonding pair of electrons. the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. why does electronegativity fall as. Hydrogen Chlorine Electronegativity.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Hydrogen Chlorine Electronegativity Find out the electronegativity of. learn how electronegativity measures the tendency of an atom to attract electrons in a bond and determines the polarity of covalent bonds. calculate the type of bond between elements based on their electronegativity values using this tool. why does electronegativity fall as you go down a group? Think of hydrogen fluoride and. Hydrogen Chlorine Electronegativity.
From dxolxdofb.blob.core.windows.net
Electronegativity Chlorine And Bromine at Tammy Parker blog Hydrogen Chlorine Electronegativity the electronegativity of hydrogen (h) is 2.18 and that of oxygen is 3.44. 119 rows find the electronegativity values of all the elements in a table, from hydrogen to lawrencium. Think of hydrogen fluoride and hydrogen chloride. learn how electronegativity determines the type of bond between atoms, from nonpolar covalent to ionic. why does electronegativity fall. Hydrogen Chlorine Electronegativity.