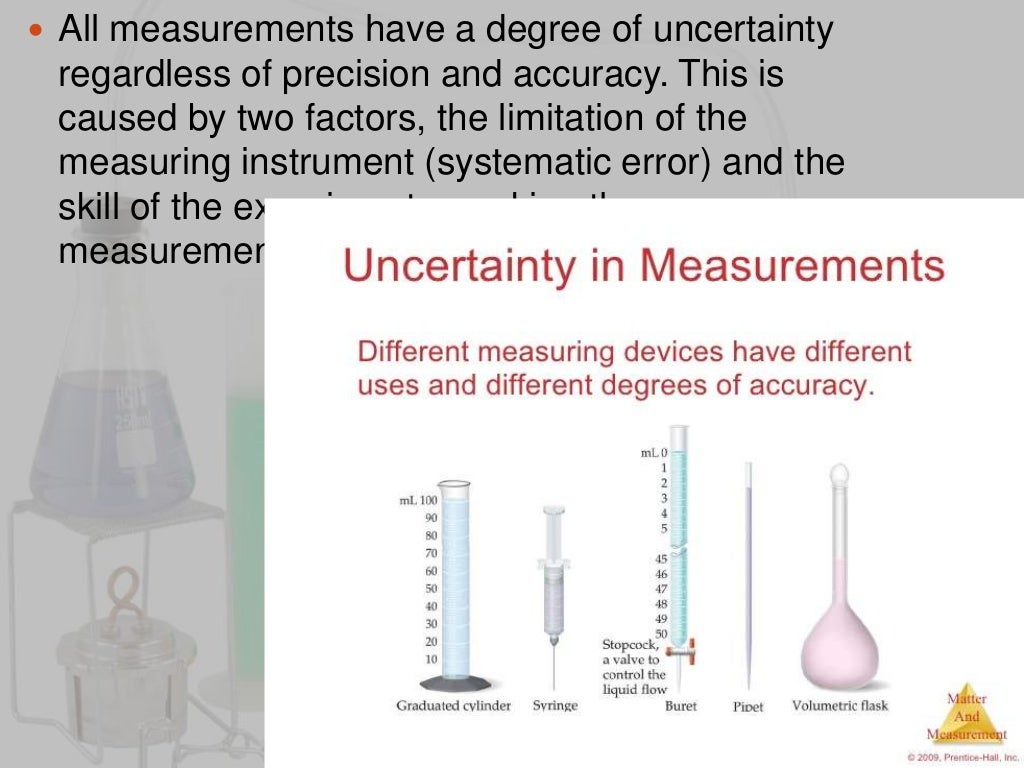
An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg . A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties in the measurements used to calculate that. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. It is given that, the weight of the compound = 10 g = 10000 mg. Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. Smaller the quantity to be. Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. We know that, 1 gram = 1000 milligram. The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et.
from www.slideshare.net
It is given that, the weight of the compound = 10 g = 10000 mg. The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et. Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. Smaller the quantity to be. We know that, 1 gram = 1000 milligram. A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties in the measurements used to calculate that.
Importance of uncertainty of measurement in chemistry
An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. It is given that, the weight of the compound = 10 g = 10000 mg. A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties in the measurements used to calculate that. Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. Smaller the quantity to be. We know that, 1 gram = 1000 milligram. The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et. Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load.
From chemistnotes.com
Analytical Balance Definition, Principle, and Reliable uses An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et. We know that, 1 gram = 1000 milligram. Smaller the quantity to be. Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. A propagation of uncertainty allows. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From askfilo.com
An analytical balance has uncertainity in measurement equal to ±1mg. Then.. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Smaller the quantity to be. Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. We know that, 1 gram = 1000 milligram. The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et. Chemists describe the estimated degree. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.labster.com
5 Ways to Make Measurements and Uncertainty Approachable for Students An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg We know that, 1 gram = 1000 milligram. Smaller the quantity to be. A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties in the measurements used to calculate that. It is given that, the weight of the compound = 10 g = 10000 mg. The uncertainty in measurement is expressed in terms of. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.pinterest.com
Postanalytical Quality Clinicians and Measurement Uncertainty An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. It is given that, the weight of the compound = 10 g = 10000 mg. Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. A propagation of. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.researchgate.net
Uncertainty measurement on Balance. Download Scientific Diagram An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Smaller the quantity to be. A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties in the measurements used to calculate that. Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. We know that, 1 gram = 1000 milligram. It is. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.isobudgets.com
How to Calculate Measurement Uncertainty in Chemistry isobudgets An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg It is given that, the weight of the compound = 10 g = 10000 mg. Smaller the quantity to be. Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From chem.libretexts.org
2.1 Measurements in Analytical Chemistry Chemistry LibreTexts An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. We know that, 1 gram = 1000 milligram. The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et. Chemists describe the estimated degree of error in. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.the-mad-scientist.net
Uncertainty in Measurement The!Mad!Scientist! An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et. We know that, 1 gram = 1000 milligram. A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties in the measurements used to calculate that. Uncertainty of analytical balances refers to the. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From hxehjpccn.blob.core.windows.net
Uncertainty Of An Analytical Balance at Kimberly Czapla blog An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. We know that, 1 gram = 1000 milligram. A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties in the measurements used to calculate that. Smaller the quantity to be. Chemists describe the estimated. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From brainly.in
An analytic balance has uncertainty in measurement equal to ±1 mg. Then An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et. Smaller the quantity to be. Uncertainty of analytical balances refers to the range of potential errors or deviations. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From boisestate.pressbooks.pub
1.5 Measurement Uncertainty, Accuracy, and Precision General An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties in the measurements used to calculate that. It is given that, the weight of the compound = 10 g = 10000 mg. The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et.. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.slideserve.com
PPT Uncertainties in Measurement PowerPoint Presentation, free An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Smaller the quantity to be. It is given that, the weight of the compound = 10 g = 10000 mg. Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. We know that, 1 gram = 1000 milligram. A propagation of uncertainty allows us to estimate the uncertainty. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From microbeonline.com
Analytical Balance Parts, Principle, and Applications • Microbe Online An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg It is given that, the weight of the compound = 10 g = 10000 mg. Smaller the quantity to be. A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties in the measurements used to calculate that. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.cheimika.it
Analytical balance 300 g 0 1 mg An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties in the measurements used to calculate that. We know that, 1 gram = 1000 milligram. It is given that, the weight of. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.pikpng.com
Analytical Balances Analytical Balance 0.1 Mg Clipart Large Size An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. Smaller the quantity to be. Uncertainty of analytical balances refers to the. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.researchgate.net
(PDF) Quantifying Uncertainty in Analytical Measurement (QUAM) An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg It is given that, the weight of the compound = 10 g = 10000 mg. Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. Smaller the quantity to be. A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties in the measurements used. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.youtube.com
11.1 Absolute and percentage uncertainties YouTube An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties in the measurements used to calculate that. Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. It is given that, the weight of the compound = 10 g = 10000 mg.. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.youtube.com
Uncertainty in Measurements YouTube An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et. It is given that, the weight of the compound = 10 g = 10000 mg. Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. Chemists describe the. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.youtube.com
Uncertainty in Measurement YouTube An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. It is given that, the weight of the compound = 10 g. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.indiamart.com
KingLab Internal 0.0001g KAB 224 Analytical Balance Machine, Capacity An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et. We know that, 1 gram = 1000 milligram. Smaller the quantity to be. A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties in the measurements used to calculate that. Uncertainty of. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.wasyresearch.com
Measurement uncertainty estimation Spreadsheets method An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et. We know that, 1 gram = 1000 milligram. Smaller the. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From ussolid.com
Analytical Balance U.S. Solid An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. Smaller the quantity to be. The uncertainty in measurement is. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From chemistnotes.com
Analytical Balance Definition, Principle, and Reliable uses An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et. Smaller the quantity to be. A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties in the measurements used to calculate that. We know that, 1 gram = 1000 milligram. Chapter 2.1.7. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.youtube.com
Calculating uncertainties 1 YouTube An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. Smaller the quantity to be. We know that, 1 gram = 1000. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From askfilo.com
An analytical balance has uncertainity in measurement equal to ±1mg. Then.. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. We know that, 1 gram = 1000 milligram. The uncertainty in measurement is expressed in terms of percentage. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From hxehjpccn.blob.core.windows.net
Uncertainty Of An Analytical Balance at Kimberly Czapla blog An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. Uncertainty of analytical balances refers to the range of potential. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.scribd.com
Measurement Uncertainty Analysis For The Calibration of Analytical An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. It is given that, the weight of the compound = 10 g = 10000 mg. Smaller the quantity. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.youtube.com
Measurement Uncertainty IB Physics YouTube An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. We know that, 1 gram = 1000 milligram. Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. Chapter 2.1.7. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.pinterest.com
Analytical Balance Definition, Principle, Parts, Types, Examples An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et. We know that, 1 gram = 1000 milligram. Chemists describe the estimated degree of error in. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.slideserve.com
PPT Chem101 Chapter 01 PowerPoint Presentation, free download ID An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. It is given that, the weight of the compound = 10 g. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.wasyresearch.com
Five reasons why measurement uncertainty is important An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et. It is given that, the weight of the compound = 10 g = 10000 mg. We know that, 1 gram = 1000 milligram. Chemists describe the estimated degree of error in a measurement as the uncertainty of the. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.youtube.com
Analytical Balance How to accurately weight Part 1 YouTube An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g., 150 ± 1 %, et. A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.youtube.com
Percent Uncertainty In Measurement YouTube An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. Chemists describe the estimated degree of error in a measurement as the uncertainty of the measurement, and they are careful to report all measured values using only significant. The uncertainty in measurement is expressed in terms of percentage by putting. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From www.slideshare.net
Importance of uncertainty of measurement in chemistry An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Smaller the quantity to be. Uncertainty of analytical balances refers to the range of potential errors or deviations that can occur during the weighing process, resulting in. We know that, 1 gram = 1000 milligram. Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. The uncertainty in measurement is. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.
From brainly.in
An analytic balance has uncertainty in measurement equal to ±1 mg. Then An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg Chapter 2.1.7 explicitly allows repeatability and sensitivity tests to be performed with the weighing vessel or a comparable tare load. A propagation of uncertainty allows us to estimate the uncertainty in a result from the uncertainties in the measurements used to calculate that. The uncertainty in measurement is expressed in terms of percentage by putting ± sign before it, e.g.,. An Analytical Balance Has Uncertainty In Measurement Equal To Plus Minus 1 Mg.