Acid Base Titration Na2Co3 And Hcl . for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the. This process is known as standardising the hydrochloric acid. today, you will use it to find the concentration of dilute hydrochloric acid by titration. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. To determine the composition of the following mixture by double indicator method: titration of na₂co₃ with hcl. See example 9.1 and check. Which indicator would be suitable for the titration of:
from www.tutormyself.com
See example 9.1 and check. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. titration of na₂co₃ with hcl. Which indicator would be suitable for the titration of: today, you will use it to find the concentration of dilute hydrochloric acid by titration. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. To determine the composition of the following mixture by double indicator method: This process is known as standardising the hydrochloric acid. for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the.
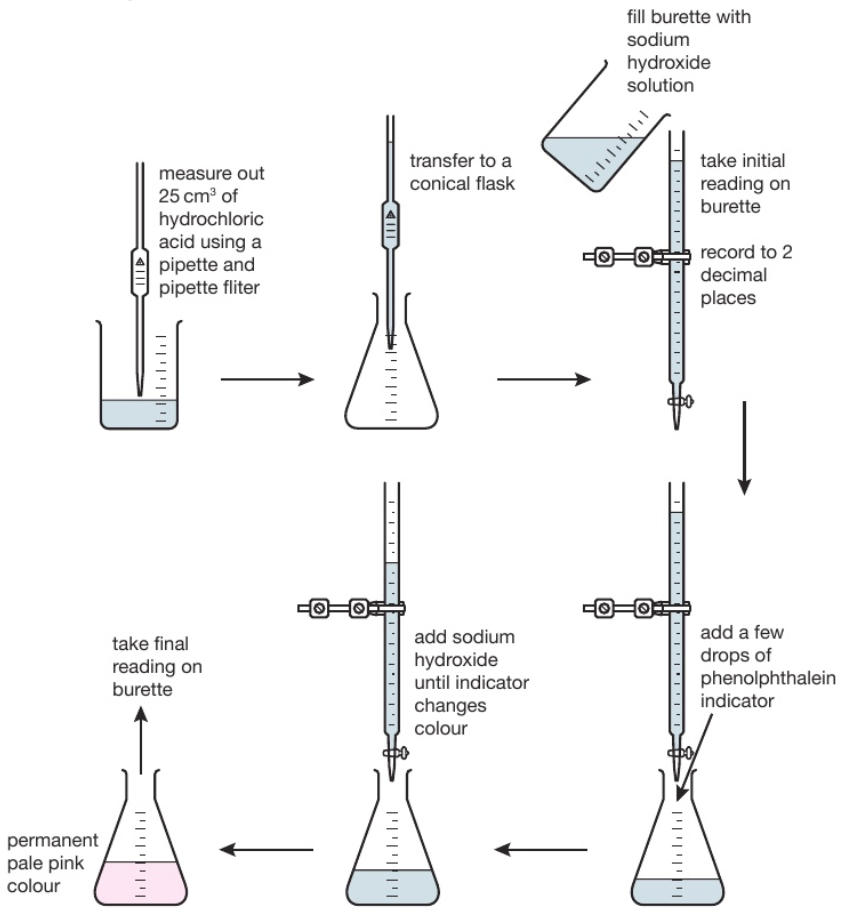
233 (Triple only) describe how to carry out an acidalkali titration
Acid Base Titration Na2Co3 And Hcl for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the. This process is known as standardising the hydrochloric acid. See example 9.1 and check. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. To determine the composition of the following mixture by double indicator method: Which indicator would be suitable for the titration of: today, you will use it to find the concentration of dilute hydrochloric acid by titration. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. titration of na₂co₃ with hcl. for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the.
From www.chegg.com
Solved Lab 12 Acid Base Titration Report Part 1 Acid Base Titration Na2Co3 And Hcl To determine the composition of the following mixture by double indicator method: when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. See example 9.1 and check. This process is known as standardising the hydrochloric acid. for the titration of a monoprotic strong acid (hcl). Acid Base Titration Na2Co3 And Hcl.
From opportunities.alumdev.columbia.edu
😱 Na2co3 hcl titration. [Solved] Titration of Na2CO3 against HCl. 2022 Acid Base Titration Na2Co3 And Hcl This process is known as standardising the hydrochloric acid. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. To determine the composition of the following mixture by double indicator method: Which indicator would be suitable for the titration of: for the titration of a. Acid Base Titration Na2Co3 And Hcl.
From www.myxxgirl.com
In An Acidbase Titration M Hcl Solution Was Added Class Chemistry Cbse Acid Base Titration Na2Co3 And Hcl This process is known as standardising the hydrochloric acid. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. today, you will use it to find the concentration of dilute hydrochloric acid by titration. See example 9.1 and check. Which indicator would be suitable for the titration of: To determine. Acid Base Titration Na2Co3 And Hcl.
From tukioka-clinic.com
😂 Titration of naoh and na2co3 with hcl. Titration Of Hcl And Na2co3 Acid Base Titration Na2Co3 And Hcl today, you will use it to find the concentration of dilute hydrochloric acid by titration. titration of na₂co₃ with hcl. This process is known as standardising the hydrochloric acid. See example 9.1 and check. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl.. Acid Base Titration Na2Co3 And Hcl.
From www.scribd.com
Stdz of HCL With NA2CO3 Titration Hydrochloric Acid Acid Base Titration Na2Co3 And Hcl for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the. To determine the composition of the following mixture by double indicator method: This process is known as standardising the hydrochloric acid. when you add a hydrochloric acid (hcl) solution to a solution. Acid Base Titration Na2Co3 And Hcl.
From www.chegg.com
Solved Experiment (?)B Standardization of HCl with Na2CO3 Acid Base Titration Na2Co3 And Hcl In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Which indicator would be suitable for the titration of: when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. today, you will use it to find. Acid Base Titration Na2Co3 And Hcl.
From www.youtube.com
Acid Base Equilibria Weak Acid Strong Base Titration. YouTube Acid Base Titration Na2Co3 And Hcl This process is known as standardising the hydrochloric acid. today, you will use it to find the concentration of dilute hydrochloric acid by titration. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh),. Acid Base Titration Na2Co3 And Hcl.
From analiticaderetail.com
Becslés férfi sarkantyú titration calculation steps Negyedkör keresztül Acid Base Titration Na2Co3 And Hcl when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the. In the overview to this chapter we noted that. Acid Base Titration Na2Co3 And Hcl.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Acid Base Titration Na2Co3 And Hcl when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. To determine the composition of the following mixture by double indicator method: This process is known as standardising the hydrochloric acid. today, you will use it to find the concentration of dilute hydrochloric acid by. Acid Base Titration Na2Co3 And Hcl.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Acid Base Titration Na2Co3 And Hcl To determine the composition of the following mixture by double indicator method: titration of na₂co₃ with hcl. This process is known as standardising the hydrochloric acid. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. for the titration of a monoprotic strong acid. Acid Base Titration Na2Co3 And Hcl.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Acid Base Titration Na2Co3 And Hcl today, you will use it to find the concentration of dilute hydrochloric acid by titration. See example 9.1 and check. for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the. titration of na₂co₃ with hcl. when you add a hydrochloric. Acid Base Titration Na2Co3 And Hcl.
From www.tessshebaylo.com
Chemical Equation For Sodium Carbonate And Hydrochloric Acid Tessshebaylo Acid Base Titration Na2Co3 And Hcl titration of na₂co₃ with hcl. This process is known as standardising the hydrochloric acid. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. Which indicator would be suitable for the titration of: See example 9.1 and check. today, you will use it to. Acid Base Titration Na2Co3 And Hcl.
From slideplayer.com
Titration Colour Changes ppt download Acid Base Titration Na2Co3 And Hcl when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. titration of na₂co₃ with hcl. for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the. In the overview. Acid Base Titration Na2Co3 And Hcl.
From saylordotorg.github.io
AcidBase Titrations Acid Base Titration Na2Co3 And Hcl titration of na₂co₃ with hcl. This process is known as standardising the hydrochloric acid. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume. Acid Base Titration Na2Co3 And Hcl.
From www.youtube.com
Lab 2 (Titration of HCl with Na2CO3) YouTube Acid Base Titration Na2Co3 And Hcl today, you will use it to find the concentration of dilute hydrochloric acid by titration. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. This process is known as standardising the hydrochloric acid. for the titration of a monoprotic strong acid (hcl) with. Acid Base Titration Na2Co3 And Hcl.
From www.researchgate.net
Acidbase titration curves of the biomass pretreated with NaHCO3 sat Acid Base Titration Na2Co3 And Hcl To determine the composition of the following mixture by double indicator method: today, you will use it to find the concentration of dilute hydrochloric acid by titration. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. See example 9.1 and check. Which indicator would be suitable for the titration. Acid Base Titration Na2Co3 And Hcl.
From pressbooks.online.ucf.edu
15.7 AcidBase Titrations Chemistry Fundamentals Acid Base Titration Na2Co3 And Hcl when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. titration of na₂co₃ with hcl. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. To determine the composition of the following mixture by double indicator. Acid Base Titration Na2Co3 And Hcl.
From www.youtube.com
Titration between Na2CO3 and HCl using phenolphthalein and methyl Acid Base Titration Na2Co3 And Hcl To determine the composition of the following mixture by double indicator method: for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen. Acid Base Titration Na2Co3 And Hcl.
From wisc.pb.unizin.org
M16Q4 Titration of a Strong Acid with a Strong Base Chem 103/104 Acid Base Titration Na2Co3 And Hcl today, you will use it to find the concentration of dilute hydrochloric acid by titration. titration of na₂co₃ with hcl. To determine the composition of the following mixture by double indicator method: when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. In the. Acid Base Titration Na2Co3 And Hcl.
From www.aiophotoz.com
Difference Between Redox Titration And Acid Base Titration Images Acid Base Titration Na2Co3 And Hcl See example 9.1 and check. titration of na₂co₃ with hcl. This process is known as standardising the hydrochloric acid. for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the. To determine the composition of the following mixture by double indicator method: Which. Acid Base Titration Na2Co3 And Hcl.
From www.youtube.com
Net Ionic Equation for Na2CO3 + HCl Sodium carbonate + Hydrochloric Acid Base Titration Na2Co3 And Hcl when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. today, you will use it to find the concentration of dilute hydrochloric acid by titration. This process is known as standardising the hydrochloric acid. To determine the composition of the following mixture by double indicator. Acid Base Titration Na2Co3 And Hcl.
From www.chegg.com
Solved 1. Consider the titration of Na2CO3 with HCl to Acid Base Titration Na2Co3 And Hcl See example 9.1 and check. today, you will use it to find the concentration of dilute hydrochloric acid by titration. titration of na₂co₃ with hcl. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. To determine the composition of the following mixture by. Acid Base Titration Na2Co3 And Hcl.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Acid Base Titration Na2Co3 And Hcl today, you will use it to find the concentration of dilute hydrochloric acid by titration. titration of na₂co₃ with hcl. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3),. Acid Base Titration Na2Co3 And Hcl.
From webmis.highland.cc.il.us
AcidBase Titrations Acid Base Titration Na2Co3 And Hcl today, you will use it to find the concentration of dilute hydrochloric acid by titration. To determine the composition of the following mixture by double indicator method: This process is known as standardising the hydrochloric acid. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. See example 9.1 and. Acid Base Titration Na2Co3 And Hcl.
From www.youtube.com
L10 pH calculation of Titration of Na2CO3 vs HCl Polyequivalent Acid Base Titration Na2Co3 And Hcl for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the. today, you will use it to find the concentration of dilute hydrochloric acid by titration. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2. Acid Base Titration Na2Co3 And Hcl.
From www.youtube.com
Acid Base titration Na2CO3 vs HCl by Awadhesh Pandey YouTube Acid Base Titration Na2Co3 And Hcl when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. today, you will use it to find the concentration of dilute hydrochloric acid by titration. . Acid Base Titration Na2Co3 And Hcl.
From mungfali.com
HCl NaOH Titration Acid Base Titration Na2Co3 And Hcl In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the. titration of na₂co₃ with hcl. To determine the composition of the following mixture. Acid Base Titration Na2Co3 And Hcl.
From mungfali.com
Acid Base Titration Indicator Acid Base Titration Na2Co3 And Hcl today, you will use it to find the concentration of dilute hydrochloric acid by titration. See example 9.1 and check. titration of na₂co₃ with hcl. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. for the titration of a monoprotic strong acid (hcl) with a monobasic strong. Acid Base Titration Na2Co3 And Hcl.
From www.youtube.com
ACID BASE TITRATION IN BANGAL, অ্যাসিড ক্ষার টাইট্রেশন, Na2CO3 vs HCl Acid Base Titration Na2Co3 And Hcl This process is known as standardising the hydrochloric acid. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. Which indicator would be suitable for the titration of: titration of na₂co₃ with hcl. for the titration of a monoprotic strong acid (hcl) with a. Acid Base Titration Na2Co3 And Hcl.
From www.youtube.com
Na2CO3 + HCl Sodium Carbonate + Hydrochloric Acid YouTube Acid Base Titration Na2Co3 And Hcl This process is known as standardising the hydrochloric acid. Which indicator would be suitable for the titration of: See example 9.1 and check. today, you will use it to find the concentration of dilute hydrochloric acid by titration. for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume. Acid Base Titration Na2Co3 And Hcl.
From www.meritnation.com
Solve this Q 49 In the titration of Na2CO3 by HCl using methyl orange Acid Base Titration Na2Co3 And Hcl Which indicator would be suitable for the titration of: for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl.. Acid Base Titration Na2Co3 And Hcl.
From childhealthpolicy.vumc.org
🌈 Na2co3 hcl titration. Why is used Na2CO3 with HCl in this titration Acid Base Titration Na2Co3 And Hcl To determine the composition of the following mixture by double indicator method: Which indicator would be suitable for the titration of: titration of na₂co₃ with hcl. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. See example 9.1 and check. for the titration of a monoprotic strong acid. Acid Base Titration Na2Co3 And Hcl.
From issuu.com
4.2 Acidbase titration by Analytical Chemistry Issuu Acid Base Titration Na2Co3 And Hcl today, you will use it to find the concentration of dilute hydrochloric acid by titration. To determine the composition of the following mixture by double indicator method: Which indicator would be suitable for the titration of: In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. See example 9.1 and. Acid Base Titration Na2Co3 And Hcl.
From studylib.net
EXPERIMENT 3. ACIDBASE TITRATIONS DETERMINATION OF Acid Base Titration Na2Co3 And Hcl when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. today, you will use it to find the concentration of dilute hydrochloric acid by titration. titration of na₂co₃ with hcl. for the titration of a monoprotic strong acid (hcl) with a monobasic strong. Acid Base Titration Na2Co3 And Hcl.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Acid Base Titration Na2Co3 And Hcl Which indicator would be suitable for the titration of: for the titration of a monoprotic strong acid (hcl) with a monobasic strong base (naoh), we can calculate the volume of base needed to reach the. when you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl.. Acid Base Titration Na2Co3 And Hcl.