Dilution Of Graph . The process of decreasing the concentration of a substance with a diluent (e.g. Generate a standard curve and use the standard curve to determine the. Water) is called dilution, and this is a highly standardized. In my mind, this dilution would instantly decrease the concentration of the. How to apply dilution factors. The concentration factor is the initial volume divided by the final. The shape of the curve provides important information about what. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. Use the spectrophotometer to measure the absorbance of solutions. A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each step. The concentration decreases by the same quantity. Serial dilutions are created by taking a series of dilutions of a stock solution.
from www.chegg.com
In my mind, this dilution would instantly decrease the concentration of the. Generate a standard curve and use the standard curve to determine the. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each step. How to apply dilution factors. The concentration decreases by the same quantity. Serial dilutions are created by taking a series of dilutions of a stock solution. Use the spectrophotometer to measure the absorbance of solutions. The shape of the curve provides important information about what. Water) is called dilution, and this is a highly standardized.
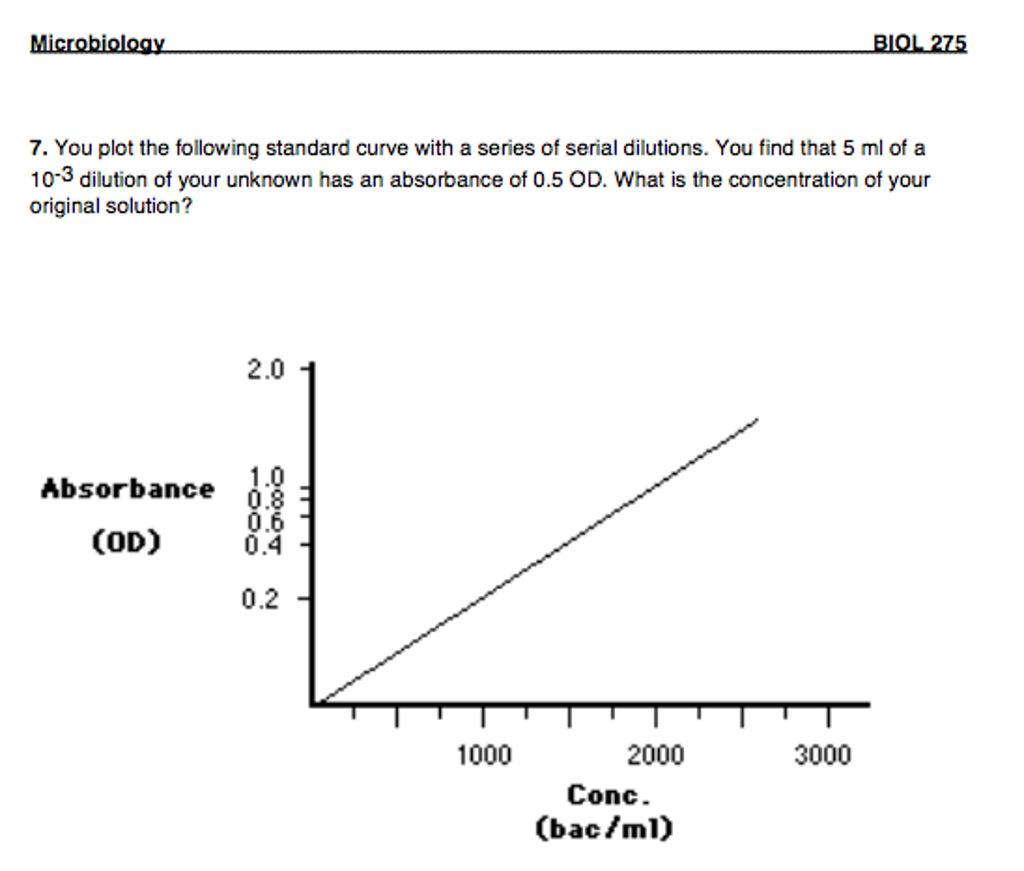
Solved You plot the following standard curve with a series
Dilution Of Graph A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each step. The concentration factor is the initial volume divided by the final. Generate a standard curve and use the standard curve to determine the. The process of decreasing the concentration of a substance with a diluent (e.g. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each step. Water) is called dilution, and this is a highly standardized. The shape of the curve provides important information about what. In my mind, this dilution would instantly decrease the concentration of the. How to apply dilution factors. Serial dilutions are created by taking a series of dilutions of a stock solution. Use the spectrophotometer to measure the absorbance of solutions. The concentration decreases by the same quantity.
From www.excel-in-science.com
Dissolution Graph Builder Excel in Science Dilution Of Graph Water) is called dilution, and this is a highly standardized. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. How to apply dilution factors. The shape of the curve provides important information about what. The process of decreasing the concentration of a substance with a diluent (e.g. A. Dilution Of Graph.
From www.youtube.com
Assess qPCR Efficiency Using a 10Fold Serial Dilution YouTube Dilution Of Graph The concentration decreases by the same quantity. The process of decreasing the concentration of a substance with a diluent (e.g. Serial dilutions are created by taking a series of dilutions of a stock solution. How to apply dilution factors. The shape of the curve provides important information about what. Plotting the ph of the solution in the flask against the. Dilution Of Graph.
From www.researchgate.net
Benchmarking of the serial dilution technique against the... Download Dilution Of Graph How to apply dilution factors. The concentration factor is the initial volume divided by the final. Generate a standard curve and use the standard curve to determine the. The concentration decreases by the same quantity. Serial dilutions are created by taking a series of dilutions of a stock solution. The process of decreasing the concentration of a substance with a. Dilution Of Graph.
From www.researchgate.net
Calibration curve of serial dilutions of Download Dilution Of Graph Serial dilutions are created by taking a series of dilutions of a stock solution. Use the spectrophotometer to measure the absorbance of solutions. The concentration factor is the initial volume divided by the final. Generate a standard curve and use the standard curve to determine the. The shape of the curve provides important information about what. Plotting the ph of. Dilution Of Graph.
From www.researchgate.net
A graph showing the optimal faecal dilution ratio (112.5) compared Dilution Of Graph The concentration decreases by the same quantity. Generate a standard curve and use the standard curve to determine the. In my mind, this dilution would instantly decrease the concentration of the. The process of decreasing the concentration of a substance with a diluent (e.g. Water) is called dilution, and this is a highly standardized. The concentration factor is the initial. Dilution Of Graph.
From ceysjldb.blob.core.windows.net
How To Graph Serial Dilutions at Linda Pike blog Dilution Of Graph In my mind, this dilution would instantly decrease the concentration of the. The shape of the curve provides important information about what. Use the spectrophotometer to measure the absorbance of solutions. The concentration factor is the initial volume divided by the final. Generate a standard curve and use the standard curve to determine the. Serial dilutions are created by taking. Dilution Of Graph.
From www.researchgate.net
Calibration curve of serial dilutions of Download Dilution Of Graph How to apply dilution factors. Serial dilutions are created by taking a series of dilutions of a stock solution. The process of decreasing the concentration of a substance with a diluent (e.g. The shape of the curve provides important information about what. The concentration factor is the initial volume divided by the final. Generate a standard curve and use the. Dilution Of Graph.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Of Graph The process of decreasing the concentration of a substance with a diluent (e.g. How to apply dilution factors. The concentration decreases by the same quantity. In my mind, this dilution would instantly decrease the concentration of the. The shape of the curve provides important information about what. Water) is called dilution, and this is a highly standardized. A serial dilution. Dilution Of Graph.
From www.researchgate.net
Serial dilution and linearity. (A), serial dilution of serum sample Dilution Of Graph A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each step. Serial dilutions are created by taking a series of dilutions of a stock solution. The concentration factor is the initial volume divided by the final. In my mind, this dilution would instantly decrease the concentration of the. Generate a standard curve and. Dilution Of Graph.
From www.researchgate.net
Calibration curve and linear dilution curve. (A) Calibration curve was Dilution Of Graph The shape of the curve provides important information about what. The concentration factor is the initial volume divided by the final. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. Water) is called dilution, and this is a highly standardized. Use the spectrophotometer to measure the absorbance of. Dilution Of Graph.
From www.researchgate.net
Dilution graphs of analyzed haematological and chemical variables in Dilution Of Graph Serial dilutions are created by taking a series of dilutions of a stock solution. Water) is called dilution, and this is a highly standardized. The shape of the curve provides important information about what. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. The concentration factor is the. Dilution Of Graph.
From www.researchgate.net
Bar graph representation of Dissolution Efficiency and Mean Dissolution Dilution Of Graph Water) is called dilution, and this is a highly standardized. The process of decreasing the concentration of a substance with a diluent (e.g. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. In my mind, this dilution would instantly decrease the concentration of the. The concentration decreases by. Dilution Of Graph.
From www.cytivalifesciences.com
Inline dilution for chromatography buffer preparation Cytiva Dilution Of Graph Use the spectrophotometer to measure the absorbance of solutions. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. Serial dilutions are created by taking a series of dilutions of a stock solution. In my mind, this dilution would instantly decrease the concentration of the. The shape of the. Dilution Of Graph.
From www.chegg.com
Solved You plot the following standard curve with a series Dilution Of Graph The concentration decreases by the same quantity. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. How to apply dilution factors. The shape of the curve provides important information about what. The concentration factor is the initial volume divided by the final. In my mind, this dilution would. Dilution Of Graph.
From www.researchgate.net
Standard curve for the log starting concentration of serial dilutions Dilution Of Graph Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. How to apply dilution factors. Water) is called dilution, and this is a highly standardized. The concentration factor is the initial volume divided by the final. Generate a standard curve and use the standard curve to determine the. The. Dilution Of Graph.
From www.researchgate.net
The dilutionresponse curves to components L, P, R. The curves were Dilution Of Graph In my mind, this dilution would instantly decrease the concentration of the. How to apply dilution factors. A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each step. The process of decreasing the concentration of a substance with a diluent (e.g. The shape of the curve provides important information about what. The concentration. Dilution Of Graph.
From www.researchgate.net
Dropletbased serial dilution of RD with various dilution factors. (A Dilution Of Graph The shape of the curve provides important information about what. Generate a standard curve and use the standard curve to determine the. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. The process of decreasing the concentration of a substance with a diluent (e.g. The concentration decreases by. Dilution Of Graph.
From chart-studio.plotly.com
Absorbance vs Dilution factor scatter chart made by Bigkendog plotly Dilution Of Graph The concentration factor is the initial volume divided by the final. A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each step. Water) is called dilution, and this is a highly standardized. The process of decreasing the concentration of a substance with a diluent (e.g. Generate a standard curve and use the standard. Dilution Of Graph.
From www.researchgate.net
Standard curve of 10fold serial dilutions (6.8 × 10 50.68 gene Dilution Of Graph Serial dilutions are created by taking a series of dilutions of a stock solution. Generate a standard curve and use the standard curve to determine the. The concentration decreases by the same quantity. The process of decreasing the concentration of a substance with a diluent (e.g. Plotting the ph of the solution in the flask against the amount of acid. Dilution Of Graph.
From www.researchgate.net
Graph showing dissolution profile of metronidazole tablets containing Dilution Of Graph In my mind, this dilution would instantly decrease the concentration of the. The concentration factor is the initial volume divided by the final. Serial dilutions are created by taking a series of dilutions of a stock solution. The process of decreasing the concentration of a substance with a diluent (e.g. A serial dilution is a series of dilutions made sequentially,. Dilution Of Graph.
From www.researchgate.net
Critical micelle dilution graph Download Scientific Diagram Dilution Of Graph The shape of the curve provides important information about what. The concentration factor is the initial volume divided by the final. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. Serial dilutions are created by taking a series of dilutions of a stock solution. The concentration decreases by. Dilution Of Graph.
From www.researchgate.net
Absorbance vs concentration graph at 462 nm, 0100 dilutions of Dilution Of Graph Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. How to apply dilution factors. Use the spectrophotometer to measure the absorbance of solutions. A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each step. The concentration decreases by the same quantity.. Dilution Of Graph.
From www.researchgate.net
Serial dilution of positive sample’s graphs. The dilution 1, 1/10 Dilution Of Graph How to apply dilution factors. Water) is called dilution, and this is a highly standardized. In my mind, this dilution would instantly decrease the concentration of the. Use the spectrophotometer to measure the absorbance of solutions. The concentration factor is the initial volume divided by the final. The shape of the curve provides important information about what. A serial dilution. Dilution Of Graph.
From ceysjldb.blob.core.windows.net
How To Graph Serial Dilutions at Linda Pike blog Dilution Of Graph The process of decreasing the concentration of a substance with a diluent (e.g. A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each step. The concentration decreases by the same quantity. Generate a standard curve and use the standard curve to determine the. In my mind, this dilution would instantly decrease the concentration. Dilution Of Graph.
From www.researchgate.net
Serial dilution plot. Each point in the serial dilution plot is Dilution Of Graph The concentration factor is the initial volume divided by the final. Generate a standard curve and use the standard curve to determine the. The process of decreasing the concentration of a substance with a diluent (e.g. How to apply dilution factors. The concentration decreases by the same quantity. Water) is called dilution, and this is a highly standardized. In my. Dilution Of Graph.
From www.researchgate.net
Dilution model compared to fast, slow and average dilution rates during Dilution Of Graph How to apply dilution factors. The concentration factor is the initial volume divided by the final. The concentration decreases by the same quantity. Water) is called dilution, and this is a highly standardized. The shape of the curve provides important information about what. A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each. Dilution Of Graph.
From www.researchgate.net
Parallelism between the standard curve and serial dilutions of pooled Dilution Of Graph Serial dilutions are created by taking a series of dilutions of a stock solution. The process of decreasing the concentration of a substance with a diluent (e.g. The concentration decreases by the same quantity. In my mind, this dilution would instantly decrease the concentration of the. The concentration factor is the initial volume divided by the final. Generate a standard. Dilution Of Graph.
From www.researchgate.net
b. Graph depicting PDA peak area data from the serial dilution of CMP Dilution Of Graph The concentration factor is the initial volume divided by the final. The shape of the curve provides important information about what. Generate a standard curve and use the standard curve to determine the. Serial dilutions are created by taking a series of dilutions of a stock solution. The concentration decreases by the same quantity. In my mind, this dilution would. Dilution Of Graph.
From www.researchgate.net
Graph of dilution broth test against S. aureus, E. coli, and E Dilution Of Graph The concentration decreases by the same quantity. How to apply dilution factors. Water) is called dilution, and this is a highly standardized. Serial dilutions are created by taking a series of dilutions of a stock solution. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. A serial dilution. Dilution Of Graph.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Dilution Of Graph The shape of the curve provides important information about what. The concentration factor is the initial volume divided by the final. A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each step. Use the spectrophotometer to measure the absorbance of solutions. Water) is called dilution, and this is a highly standardized. How to. Dilution Of Graph.
From www.researchgate.net
Graph illustrating optimal antigen concentration and antibody dilution Dilution Of Graph Use the spectrophotometer to measure the absorbance of solutions. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. Water) is called dilution, and this is a highly standardized. Generate a standard curve and use the standard curve to determine the. A serial dilution is a series of dilutions. Dilution Of Graph.
From chemistnotes.com
Variation of conductance with dilution Chemistry Notes Dilution Of Graph Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. In my mind, this dilution would instantly decrease the concentration of the. Water) is called dilution, and this is a highly standardized. The process of decreasing the concentration of a substance with a diluent (e.g. The concentration decreases by. Dilution Of Graph.
From bio.libretexts.org
15 Determination of Bacterial Numbers Biology LibreTexts Dilution Of Graph Use the spectrophotometer to measure the absorbance of solutions. Plotting the ph of the solution in the flask against the amount of acid or base added produces a titration curve. Serial dilutions are created by taking a series of dilutions of a stock solution. A serial dilution is a series of dilutions made sequentially, using the same dilution factor for. Dilution Of Graph.
From www.researchgate.net
Dilution graphs of analyzed haematological and chemical variables in Dilution Of Graph The concentration decreases by the same quantity. The shape of the curve provides important information about what. In my mind, this dilution would instantly decrease the concentration of the. Water) is called dilution, and this is a highly standardized. Use the spectrophotometer to measure the absorbance of solutions. How to apply dilution factors. A serial dilution is a series of. Dilution Of Graph.
From www.researchgate.net
Real time PCR and linear correlation of 10fold serial dilution of Dilution Of Graph The concentration decreases by the same quantity. The shape of the curve provides important information about what. The concentration factor is the initial volume divided by the final. Use the spectrophotometer to measure the absorbance of solutions. A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each step. How to apply dilution factors.. Dilution Of Graph.