Medical Devices Defined By Smda . “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. In 1997, the smda has renamed the fda modernization act (fdama). The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical devices to report any. Medical devices, as regulated by the fda, were originally defined in the fd&c act as: The safe medical devices act (smda) is a u.s. How does the smda define medical devices? Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities and to report any adverse events associated with a medical device to the fda.
from unifymedsupply.com
Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. In 1997, the smda has renamed the fda modernization act (fdama). How does the smda define medical devices? The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical devices to report any. The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities and to report any adverse events associated with a medical device to the fda. The safe medical devices act (smda) is a u.s. “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” Medical devices, as regulated by the fda, were originally defined in the fd&c act as: In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the.
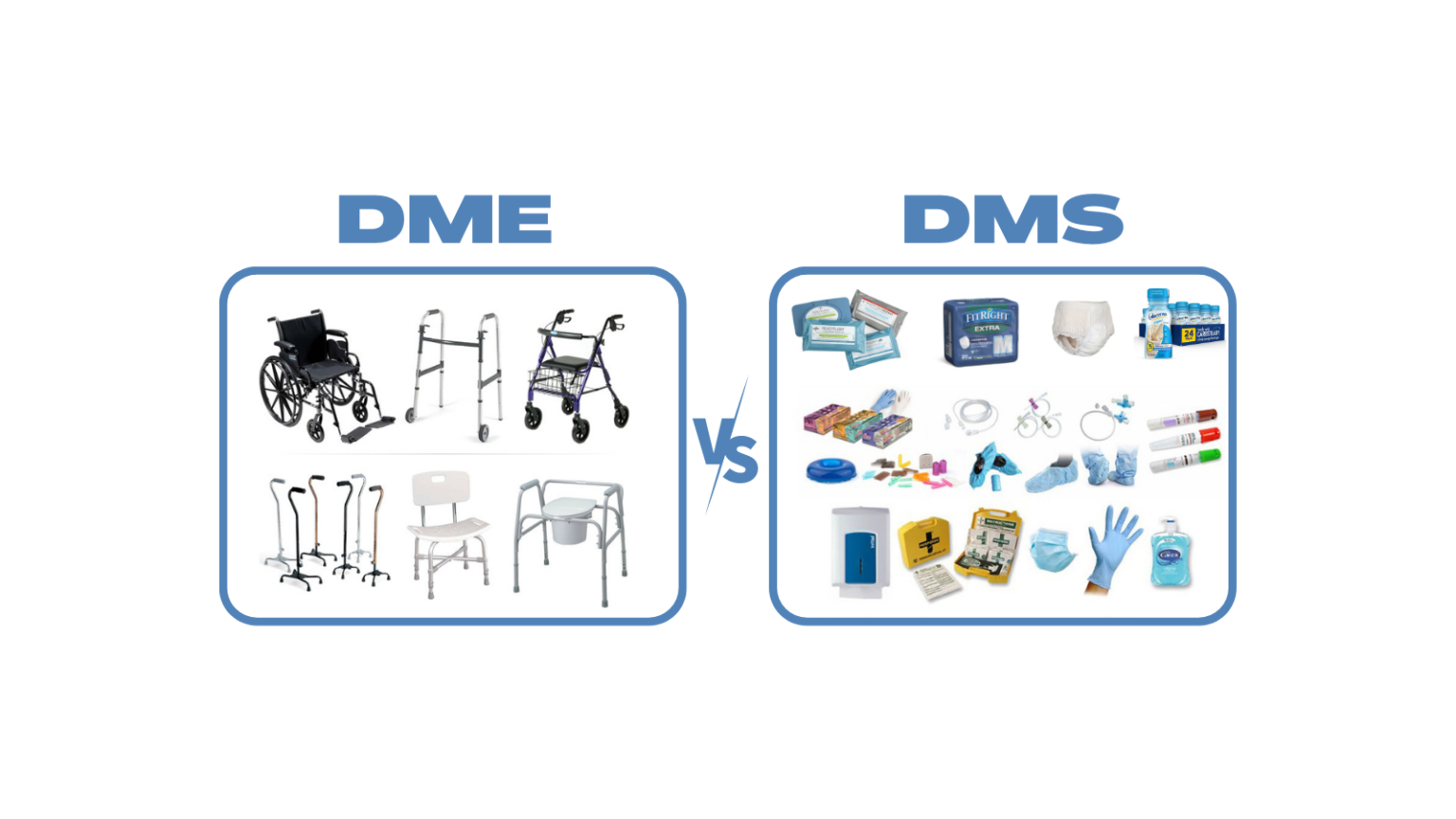
Difference between Medical Devices and Medical Consumables
Medical Devices Defined By Smda In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities and to report any adverse events associated with a medical device to the fda. Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. The safe medical devices act (smda) is a u.s. In 1997, the smda has renamed the fda modernization act (fdama). How does the smda define medical devices? The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical devices to report any. “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Medical devices, as regulated by the fda, were originally defined in the fd&c act as:
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By Smda In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities and to report. Medical Devices Defined By Smda.
From de.slideshare.net
Medical device definition Medical Devices Defined By Smda In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. In 1997, the smda has renamed the fda modernization act (fdama). The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical devices to report any. How does the. Medical Devices Defined By Smda.
From es.slideshare.net
Regulation of Medical Devices in US Medical Devices Defined By Smda The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical devices to report any. Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the.. Medical Devices Defined By Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By Smda The safe medical devices act (smda) is a u.s. Medical devices, as regulated by the fda, were originally defined in the fd&c act as: Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. The safe. Medical Devices Defined By Smda.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group Medical Devices Defined By Smda In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. How does the smda define medical devices? The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical devices to report any. “any instrument, machine, contrivance, implant, in vitro. Medical Devices Defined By Smda.
From www.slideserve.com
PPT Medical Equipment PowerPoint Presentation, free download ID4432311 Medical Devices Defined By Smda The safe medical devices act (smda) is a u.s. In 1997, the smda has renamed the fda modernization act (fdama). In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. The safe medical devices act (smda). Medical Devices Defined By Smda.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Devices Defined By Smda “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. The safe medical devices act (smda) is a u.s. The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities. Medical Devices Defined By Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By Smda Medical devices, as regulated by the fda, were originally defined in the fd&c act as: In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities and to report any adverse events associated. Medical Devices Defined By Smda.
From www.youtube.com
What are medical devices? YouTube Medical Devices Defined By Smda In 1997, the smda has renamed the fda modernization act (fdama). Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities and to report any adverse events associated with a medical device to the fda. In the 1960s. Medical Devices Defined By Smda.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Devices Defined By Smda The safe medical devices act (smda) is a u.s. Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical devices to report any. “any instrument, machine, contrivance, implant, in vitro reagent that's intended to. Medical Devices Defined By Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By Smda The safe medical devices act (smda) is a u.s. How does the smda define medical devices? In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” Law enacted in 1990 to. Medical Devices Defined By Smda.
From www.slideserve.com
PPT Medical Devices PowerPoint Presentation, free download ID991833 Medical Devices Defined By Smda In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. How does the smda define medical devices? The safe medical devices act (smda) is a u.s. In 1997, the smda has renamed the fda modernization act (fdama). Law enacted in 1990 to enhance the fda's authority to regulate medical devices. Medical Devices Defined By Smda.
From slideplayer.com
Ensuring a Safe Environment at Work ppt download Medical Devices Defined By Smda The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities and to report any adverse events associated with a medical device to the fda. Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. Medical devices, as regulated by the fda, were originally defined in the fd&c act. Medical Devices Defined By Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By Smda The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical devices to report any. In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Law enacted in 1990 to enhance the fda's authority to regulate medical devices and.. Medical Devices Defined By Smda.
From www.qualio.com
The complete guide to SaMD (software as a medical device) Medical Devices Defined By Smda The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical devices to report any. The safe medical devices act (smda) is a u.s. Medical devices, as regulated by the fda, were originally defined in the fd&c act as: Law enacted in 1990 to enhance the fda's authority to. Medical Devices Defined By Smda.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Devices Defined By Smda How does the smda define medical devices? The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities and to report any adverse events associated with a medical device to the fda. Medical devices, as regulated by the fda, were originally defined in the fd&c act as: Law enacted in 1990 to enhance. Medical Devices Defined By Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By Smda “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. Medical devices, as regulated by the fda,. Medical Devices Defined By Smda.
From www.slideserve.com
PPT Overview of FDA Regulation of Devices & Diagnostics PowerPoint Medical Devices Defined By Smda The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities and to report any adverse events associated with a medical device to the fda. The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical devices to report any. The safe. Medical Devices Defined By Smda.
From www.collidu.com
Software as a Medical Device (SaMD) PowerPoint and Google Slides Medical Devices Defined By Smda The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities and to report any adverse events associated with a medical device to the fda. Medical devices, as regulated by the fda, were originally defined in the fd&c act as: “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure,. Medical Devices Defined By Smda.
From www.qmswrapper.com
What is medical devices lifecycle qmsWrapper Medical Devices Defined By Smda In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. How does the smda define medical devices? “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” The safe medical devices act of 1990 (smda) is a federal law that requires. Medical Devices Defined By Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By Smda Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. How does the smda define medical devices? In 1997, the smda has renamed the fda modernization act (fdama). “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” The safe medical devices act (smda) is a u.s.. Medical Devices Defined By Smda.
From kantify.com
Kantify Improving Human Health through Artificial Intelligence Medical Devices Defined By Smda In 1997, the smda has renamed the fda modernization act (fdama). Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. Medical devices, as regulated by the fda, were originally defined in the fd&c act as: How does the smda define medical devices? The safe medical devices act (smda) of 1990 is a federal law in. Medical Devices Defined By Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By Smda The safe medical devices act (smda) is a u.s. In 1997, the smda has renamed the fda modernization act (fdama). Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities and to report any adverse events associated with. Medical Devices Defined By Smda.
From unifymedsupply.com
Difference between Medical Devices and Medical Consumables Medical Devices Defined By Smda How does the smda define medical devices? The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical devices to report any. In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. The safe medical devices act of 1990. Medical Devices Defined By Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By Smda “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical devices to report any. In. Medical Devices Defined By Smda.
From vem-medical.com
Medical Device Manufacturing Medical Devices Defined By Smda Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” In 1997, the smda has renamed the fda modernization act (fdama). Medical devices, as regulated by the fda, were originally defined in the fd&c act as: The. Medical Devices Defined By Smda.
From www.slideserve.com
PPT The FDA Landscape AdvaMed September 2008 PowerPoint Presentation Medical Devices Defined By Smda In 1997, the smda has renamed the fda modernization act (fdama). The safe medical devices act (smda) is a u.s. “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use. Medical Devices Defined By Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By Smda The safe medical devices act (smda) is a u.s. The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical devices to report any. How does the smda define medical devices? Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. In the 1960s and. Medical Devices Defined By Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By Smda In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. In 1997, the smda has renamed the fda modernization act (fdama). “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” How does the smda define medical devices? The safe medical. Medical Devices Defined By Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By Smda Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. The safe medical devices act (smda) is a u.s. Medical devices, as regulated by the fda, were originally defined in the fd&c act as: The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities and to report any. Medical Devices Defined By Smda.
From laegemiddelstyrelsen.dk
Development of medical devices Medical Devices Defined By Smda In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. How does the smda define medical devices? Law enacted in 1990 to enhance the fda's authority to regulate medical devices and. The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities. Medical Devices Defined By Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By Smda “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” In 1997, the smda has renamed the fda modernization act (fdama). The safe medical devices act (smda) is a u.s. The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities and to report. Medical Devices Defined By Smda.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Devices Defined By Smda The safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical devices to report any. In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. The safe medical devices act of 1990 (smda) is a federal law that requires. Medical Devices Defined By Smda.
From operonstrategist.com
Software as a Medical Device (SaMD) IEC 62304 Certification Medical Devices Defined By Smda “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities and to report any adverse events associated with a medical device to the fda. In 1997, the smda has renamed the fda modernization. Medical Devices Defined By Smda.
From medicaldevicehq.com
What is a medical device? Medical Device HQ Medical Devices Defined By Smda “any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.” In the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by passing the. The safe medical devices act of 1990 (smda) is a federal law that requires manufacturers and user facilities and to report. Medical Devices Defined By Smda.