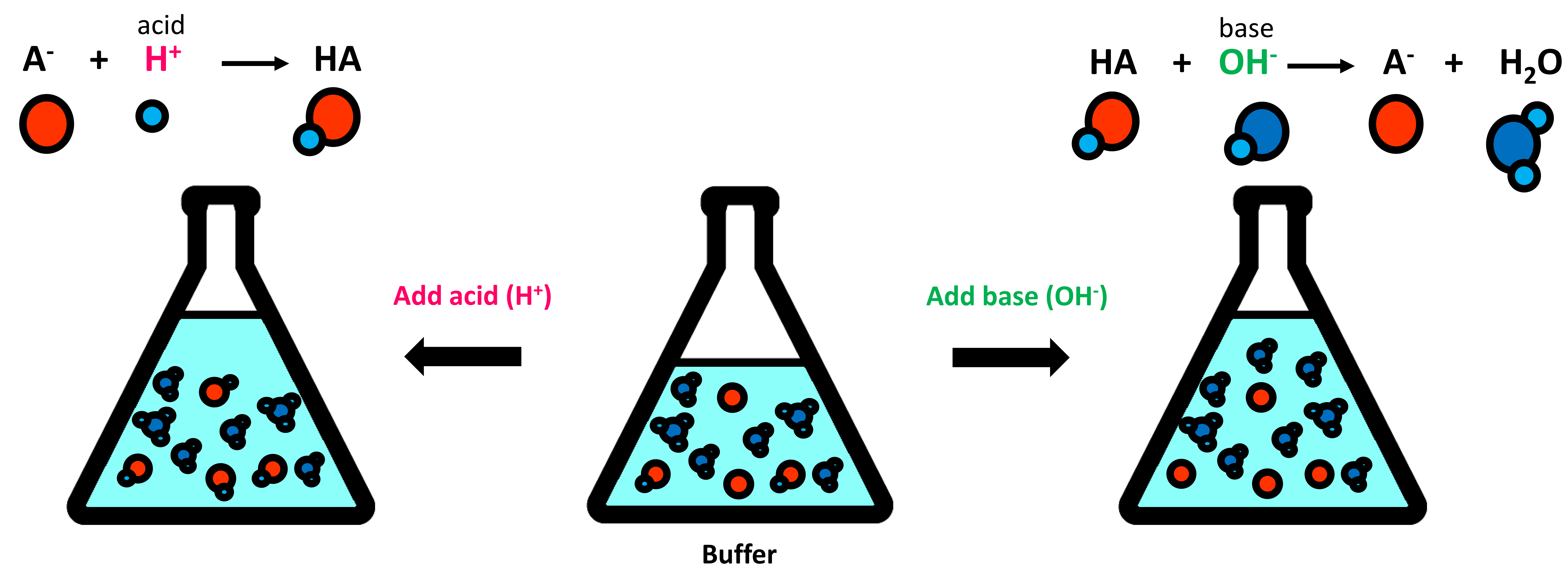
How Does Ph Affect Buffer Capacity . In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. Instead, the ability of a. Buffer solutions do not have an unlimited capacity to keep the ph relatively constant (figure 14.16). Buffers are characterized by their ph range and buffer capacity. The buffer capacity is measured experimentally at a particular ph by titration against a strong. What factors determine the effectiveness (or capacity) of a buffer? The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. The goal of a buffer is to keep the ph of a solution within a narrow range. Buffers are characterized by their ph range and buffer capacity. The useful ph range of a buffer depends on the chemical properties of the conjugate. The extent of resistance to ph change is called the buffer capacity of a solution.
from pdfprof.com
Buffers are characterized by their ph range and buffer capacity. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. The useful ph range of a buffer depends on the chemical properties of the conjugate. The buffer capacity is measured experimentally at a particular ph by titration against a strong. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. What factors determine the effectiveness (or capacity) of a buffer? The extent of resistance to ph change is called the buffer capacity of a solution. Instead, the ability of a. The goal of a buffer is to keep the ph of a solution within a narrow range. Buffer solutions do not have an unlimited capacity to keep the ph relatively constant (figure 14.16).
buffer capacity experiment procedure
How Does Ph Affect Buffer Capacity Buffer solutions do not have an unlimited capacity to keep the ph relatively constant (figure 14.16). The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. Buffers are characterized by their ph range and buffer capacity. Buffer solutions do not have an unlimited capacity to keep the ph relatively constant (figure 14.16). The useful ph range of a buffer depends on the chemical properties of the conjugate. The goal of a buffer is to keep the ph of a solution within a narrow range. The buffer capacity is measured experimentally at a particular ph by titration against a strong. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. Instead, the ability of a. Buffers are characterized by their ph range and buffer capacity. What factors determine the effectiveness (or capacity) of a buffer? The extent of resistance to ph change is called the buffer capacity of a solution.
From facts.net
13 Enigmatic Facts About Buffer Capacity How Does Ph Affect Buffer Capacity Buffers are characterized by their ph range and buffer capacity. What factors determine the effectiveness (or capacity) of a buffer? The buffer capacity is measured experimentally at a particular ph by titration against a strong. The extent of resistance to ph change is called the buffer capacity of a solution. Buffers are characterized by their ph range and buffer capacity.. How Does Ph Affect Buffer Capacity.
From animalia-life.club
Phosphate Buffer System Equation How Does Ph Affect Buffer Capacity What factors determine the effectiveness (or capacity) of a buffer? Buffers are characterized by their ph range and buffer capacity. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. Buffers are. How Does Ph Affect Buffer Capacity.
From www.researchgate.net
Buffering capacity β of (a) water, (b) ammonia or ammonium, and (c How Does Ph Affect Buffer Capacity Instead, the ability of a. Buffers are characterized by their ph range and buffer capacity. Buffer solutions do not have an unlimited capacity to keep the ph relatively constant (figure 14.16). The goal of a buffer is to keep the ph of a solution within a narrow range. The useful ph range of a buffer depends on the chemical properties. How Does Ph Affect Buffer Capacity.
From www.slideserve.com
PPT Buffers & Buffer Capacity PowerPoint Presentation ID5949383 How Does Ph Affect Buffer Capacity The goal of a buffer is to keep the ph of a solution within a narrow range. The buffer capacity is measured experimentally at a particular ph by titration against a strong. Buffer solutions do not have an unlimited capacity to keep the ph relatively constant (figure 14.16). The buffer capacity is the amount of acid or base that can. How Does Ph Affect Buffer Capacity.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded How Does Ph Affect Buffer Capacity What factors determine the effectiveness (or capacity) of a buffer? In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. The buffer capacity is the amount of acid or base that can. How Does Ph Affect Buffer Capacity.
From www.slideserve.com
PPT Chapter 5 Water and Seawater PowerPoint Presentation, free How Does Ph Affect Buffer Capacity What factors determine the effectiveness (or capacity) of a buffer? Buffers are characterized by their ph range and buffer capacity. The extent of resistance to ph change is called the buffer capacity of a solution. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1. How Does Ph Affect Buffer Capacity.
From byjus.com
Buffer Region What is a Buffer Region, Relationship between Titration How Does Ph Affect Buffer Capacity What factors determine the effectiveness (or capacity) of a buffer? The goal of a buffer is to keep the ph of a solution within a narrow range. Buffers are characterized by their ph range and buffer capacity. The extent of resistance to ph change is called the buffer capacity of a solution. Instead, the ability of a. Buffers are characterized. How Does Ph Affect Buffer Capacity.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube How Does Ph Affect Buffer Capacity The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. The useful ph range of a buffer depends on the chemical properties of the conjugate. The extent of resistance to ph change is called the buffer capacity of a solution. The goal of. How Does Ph Affect Buffer Capacity.
From sukhrajsiara.blogspot.com
25+ Buffer Capacity Calculation SukhrajSiara How Does Ph Affect Buffer Capacity The extent of resistance to ph change is called the buffer capacity of a solution. The goal of a buffer is to keep the ph of a solution within a narrow range. Instead, the ability of a. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added. How Does Ph Affect Buffer Capacity.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free How Does Ph Affect Buffer Capacity Buffers are characterized by their ph range and buffer capacity. The goal of a buffer is to keep the ph of a solution within a narrow range. The useful ph range of a buffer depends on the chemical properties of the conjugate. Buffers are characterized by their ph range and buffer capacity. Instead, the ability of a. What factors determine. How Does Ph Affect Buffer Capacity.
From users.highland.edu
Buffers How Does Ph Affect Buffer Capacity The extent of resistance to ph change is called the buffer capacity of a solution. Buffers are characterized by their ph range and buffer capacity. Instead, the ability of a. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to. How Does Ph Affect Buffer Capacity.
From www.chegg.com
Solved (a) Calculate the buffer capacity for each of the How Does Ph Affect Buffer Capacity Buffer solutions do not have an unlimited capacity to keep the ph relatively constant (figure 14.16). The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. Buffers are characterized by their ph range and buffer capacity. Instead, the ability of a. What factors. How Does Ph Affect Buffer Capacity.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded How Does Ph Affect Buffer Capacity The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. The useful ph range of a buffer depends on the chemical properties of the conjugate. Instead, the ability of a. The extent of resistance to ph change is called the buffer capacity of. How Does Ph Affect Buffer Capacity.
From www.researchgate.net
The effect of pH on the buffer capacity in wine. Download Scientific How Does Ph Affect Buffer Capacity Buffers are characterized by their ph range and buffer capacity. The goal of a buffer is to keep the ph of a solution within a narrow range. What factors determine the effectiveness (or capacity) of a buffer? In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added. How Does Ph Affect Buffer Capacity.
From www.youtube.com
Buffer range and capacity YouTube How Does Ph Affect Buffer Capacity The extent of resistance to ph change is called the buffer capacity of a solution. Instead, the ability of a. What factors determine the effectiveness (or capacity) of a buffer? The goal of a buffer is to keep the ph of a solution within a narrow range. Buffers are characterized by their ph range and buffer capacity. The buffer capacity. How Does Ph Affect Buffer Capacity.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint How Does Ph Affect Buffer Capacity The extent of resistance to ph change is called the buffer capacity of a solution. Buffers are characterized by their ph range and buffer capacity. The buffer capacity is measured experimentally at a particular ph by titration against a strong. Instead, the ability of a. The buffer capacity is the amount of acid or base that can be added to. How Does Ph Affect Buffer Capacity.
From users.highland.edu
Buffers How Does Ph Affect Buffer Capacity The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. The useful ph range of a buffer depends on the chemical properties of the conjugate. Buffer solutions do not have an unlimited capacity to keep the ph relatively constant (figure 14.16). The goal. How Does Ph Affect Buffer Capacity.
From www.studypool.com
SOLUTION 4 buffering capacity Studypool How Does Ph Affect Buffer Capacity Buffers are characterized by their ph range and buffer capacity. Buffer solutions do not have an unlimited capacity to keep the ph relatively constant (figure 14.16). The useful ph range of a buffer depends on the chemical properties of the conjugate. What factors determine the effectiveness (or capacity) of a buffer? The extent of resistance to ph change is called. How Does Ph Affect Buffer Capacity.
From www.pinterest.com
Buffer Capacity definition The amount of acid or a base a buffer How Does Ph Affect Buffer Capacity Buffer solutions do not have an unlimited capacity to keep the ph relatively constant (figure 14.16). The extent of resistance to ph change is called the buffer capacity of a solution. The useful ph range of a buffer depends on the chemical properties of the conjugate. In more rigorous terms, buffer capacity is defined as the number of moles of. How Does Ph Affect Buffer Capacity.
From www.researchgate.net
Temperature dependence of the pH of histidine buffer, the pH of How Does Ph Affect Buffer Capacity The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. The useful ph range of a buffer depends on the chemical properties of the conjugate. Instead, the ability of a. In more rigorous terms, buffer capacity is defined as the number of moles. How Does Ph Affect Buffer Capacity.
From study.com
Identifying Buffer Capacity Chemistry How Does Ph Affect Buffer Capacity Buffers are characterized by their ph range and buffer capacity. The useful ph range of a buffer depends on the chemical properties of the conjugate. The buffer capacity is measured experimentally at a particular ph by titration against a strong. Instead, the ability of a. What factors determine the effectiveness (or capacity) of a buffer? The goal of a buffer. How Does Ph Affect Buffer Capacity.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free How Does Ph Affect Buffer Capacity Buffers are characterized by their ph range and buffer capacity. The extent of resistance to ph change is called the buffer capacity of a solution. The buffer capacity is measured experimentally at a particular ph by titration against a strong. Instead, the ability of a. The goal of a buffer is to keep the ph of a solution within a. How Does Ph Affect Buffer Capacity.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID720100 How Does Ph Affect Buffer Capacity Instead, the ability of a. Buffer solutions do not have an unlimited capacity to keep the ph relatively constant (figure 14.16). The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. What factors determine the effectiveness (or capacity) of a buffer? In more. How Does Ph Affect Buffer Capacity.
From www.youtube.com
Buffer capacity; making a buffer with a given pH value YouTube How Does Ph Affect Buffer Capacity The buffer capacity is measured experimentally at a particular ph by titration against a strong. Instead, the ability of a. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. The extent. How Does Ph Affect Buffer Capacity.
From kuncijawabanpdf.blogspot.com
Soal Dapar Ph 7 4 Kunci Jawaban How Does Ph Affect Buffer Capacity What factors determine the effectiveness (or capacity) of a buffer? The extent of resistance to ph change is called the buffer capacity of a solution. Buffers are characterized by their ph range and buffer capacity. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1. How Does Ph Affect Buffer Capacity.
From saylordotorg.github.io
Buffers How Does Ph Affect Buffer Capacity The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. The extent of resistance to ph change is called the buffer capacity of a solution. The buffer capacity is measured experimentally at a particular ph by titration against a strong. In more rigorous. How Does Ph Affect Buffer Capacity.
From pdfprof.com
buffer capacity experiment procedure How Does Ph Affect Buffer Capacity What factors determine the effectiveness (or capacity) of a buffer? The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. Buffers are characterized by their ph range and buffer capacity. The goal of a buffer is to keep the ph of a solution. How Does Ph Affect Buffer Capacity.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint How Does Ph Affect Buffer Capacity Buffer solutions do not have an unlimited capacity to keep the ph relatively constant (figure 14.16). The extent of resistance to ph change is called the buffer capacity of a solution. The goal of a buffer is to keep the ph of a solution within a narrow range. Instead, the ability of a. The buffer capacity is measured experimentally at. How Does Ph Affect Buffer Capacity.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts How Does Ph Affect Buffer Capacity The useful ph range of a buffer depends on the chemical properties of the conjugate. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. Buffers are characterized by their ph range and buffer capacity. Buffers are characterized by their ph range and. How Does Ph Affect Buffer Capacity.
From ppt-online.org
Solutions. Acidbase equilibrium in biological systems презентация онлайн How Does Ph Affect Buffer Capacity What factors determine the effectiveness (or capacity) of a buffer? The buffer capacity is measured experimentally at a particular ph by titration against a strong. The goal of a buffer is to keep the ph of a solution within a narrow range. The buffer capacity is the amount of acid or base that can be added to a given volume. How Does Ph Affect Buffer Capacity.
From byjus.com
Buffer capacity of buffer containing acid with pKa = 4.75 will be How Does Ph Affect Buffer Capacity Buffer solutions do not have an unlimited capacity to keep the ph relatively constant (figure 14.16). What factors determine the effectiveness (or capacity) of a buffer? The buffer capacity is measured experimentally at a particular ph by titration against a strong. The useful ph range of a buffer depends on the chemical properties of the conjugate. The buffer capacity is. How Does Ph Affect Buffer Capacity.
From www.chegg.com
Solved 1. How does the concentration of the buffer affect How Does Ph Affect Buffer Capacity What factors determine the effectiveness (or capacity) of a buffer? Buffers are characterized by their ph range and buffer capacity. The extent of resistance to ph change is called the buffer capacity of a solution. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph. How Does Ph Affect Buffer Capacity.
From www.slideserve.com
PPT THEME Acidbase equilibrium in biological systems. Buffer How Does Ph Affect Buffer Capacity Buffer solutions do not have an unlimited capacity to keep the ph relatively constant (figure 14.16). The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or. How Does Ph Affect Buffer Capacity.
From www.youtube.com
14.10 Buffers Solutions that Resist pH Change YouTube How Does Ph Affect Buffer Capacity In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. Buffers are characterized by their ph range and buffer capacity. The useful ph range of a buffer depends on the chemical properties. How Does Ph Affect Buffer Capacity.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint How Does Ph Affect Buffer Capacity The extent of resistance to ph change is called the buffer capacity of a solution. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. The goal of a buffer is to keep the ph of a solution within a narrow range. Buffers. How Does Ph Affect Buffer Capacity.