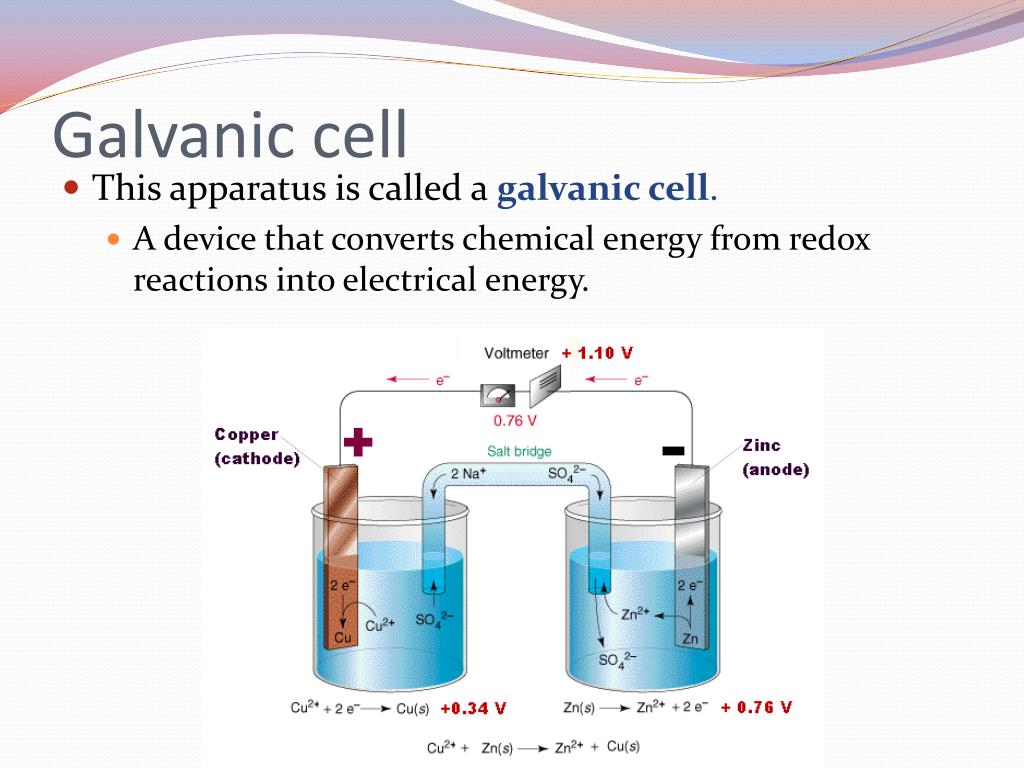
Which Energy Conversion Occurs In A Voltaic Cell . In a galvanic (voltaic) cell, the. The important parts of a voltaic cell: A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. The anode is an electrode where oxidation. Which energy conversions occurs in a operating voltaic cell. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Chemical energy to electrical energy. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction.
from www.slideserve.com
The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. Which energy conversions occurs in a operating voltaic cell. In a galvanic (voltaic) cell, the. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. The anode is an electrode where oxidation. The important parts of a voltaic cell: A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Chemical energy to electrical energy.
PPT Galvanic Cells Converting chemical energy to electrical energy
Which Energy Conversion Occurs In A Voltaic Cell Chemical energy to electrical energy. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. In a galvanic (voltaic) cell, the. Chemical energy to electrical energy. The anode is an electrode where oxidation. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. The important parts of a voltaic cell: Which energy conversions occurs in a operating voltaic cell. A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy.
From 2012books.lardbucket.org
Describing Electrochemical Cells Which Energy Conversion Occurs In A Voltaic Cell A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. In a galvanic (voltaic) cell, the. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. The important parts of a voltaic cell: Chemical energy to electrical energy.. Which Energy Conversion Occurs In A Voltaic Cell.
From www.askiitians.com
Daniell Cell Study Material for IIT JEE askIITians Which Energy Conversion Occurs In A Voltaic Cell In a galvanic (voltaic) cell, the. Chemical energy to electrical energy. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The important parts of a voltaic cell: Which energy conversions occurs in a operating voltaic cell. The galvanic cell, or called voltaic cell, is an electrochemical cell that. Which Energy Conversion Occurs In A Voltaic Cell.
From www.chegg.com
Solved 3. Which process occurs in an operating voltaic cell? Which Energy Conversion Occurs In A Voltaic Cell The anode is an electrode where oxidation. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. The important parts of a voltaic cell: Which energy conversions occurs in a operating voltaic cell. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox. Which Energy Conversion Occurs In A Voltaic Cell.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells Which Energy Conversion Occurs In A Voltaic Cell A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. In a galvanic (voltaic) cell, the. Chemical energy to electrical energy. The important parts of a voltaic cell: The anode is an electrode where oxidation. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous. Which Energy Conversion Occurs In A Voltaic Cell.
From www.slideserve.com
PPT Galvanic Cells Converting chemical energy to electrical energy Which Energy Conversion Occurs In A Voltaic Cell A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. The anode is an electrode where oxidation. Chemical energy to electrical energy. A voltaic cell (also known as a galvanic cell) is an. Which Energy Conversion Occurs In A Voltaic Cell.
From joel-chapter.blogspot.com
A Cell Contains Two Hydrogen Electrodes 35+ Pages Summary [2.8mb Which Energy Conversion Occurs In A Voltaic Cell In a galvanic (voltaic) cell, the. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. Chemical energy to electrical energy. Which energy conversions occurs in a operating voltaic cell. The anode is an electrode where oxidation. A voltaic cell is an electrochemical cell that uses a chemical reaction to. Which Energy Conversion Occurs In A Voltaic Cell.
From www.youtube.com
Emf of cell,Cell potential (Electrochemistry part 13 for CBSE class 12 Which Energy Conversion Occurs In A Voltaic Cell An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. In a galvanic (voltaic) cell, the. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A voltaic cell is an electrochemical cell that uses a chemical reaction to. Which Energy Conversion Occurs In A Voltaic Cell.
From cosmosmagazine.com
How solar cells turn sunlight into electricity Cosmos Magazine Which Energy Conversion Occurs In A Voltaic Cell Which energy conversions occurs in a operating voltaic cell. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. The galvanic cell, or called voltaic cell, is an electrochemical. Which Energy Conversion Occurs In A Voltaic Cell.
From www.researchgate.net
Block diagram of photo voltaic solar energy conversion system Which Energy Conversion Occurs In A Voltaic Cell Chemical energy to electrical energy. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. The anode is an electrode where oxidation. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A voltaic cell is an electrochemical cell. Which Energy Conversion Occurs In A Voltaic Cell.
From golifescience.com
Energy Transformation What is Bioenergetics and Free energy Which Energy Conversion Occurs In A Voltaic Cell In a galvanic (voltaic) cell, the. The anode is an electrode where oxidation. Chemical energy to electrical energy. Which energy conversions occurs in a operating voltaic cell. The important parts of a voltaic cell: A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The galvanic cell, or called. Which Energy Conversion Occurs In A Voltaic Cell.
From www.researchgate.net
Illustration of solar thermochemical energy conversion process Which Energy Conversion Occurs In A Voltaic Cell In a galvanic (voltaic) cell, the. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Chemical energy to electrical energy. The galvanic cell, or called voltaic cell, is. Which Energy Conversion Occurs In A Voltaic Cell.
From byjus.com
Name the energy transformation taking place in the following device a Which Energy Conversion Occurs In A Voltaic Cell The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. Which energy conversions occurs in a operating voltaic cell. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. A voltaic cell is an electrochemical cell that uses a. Which Energy Conversion Occurs In A Voltaic Cell.
From slideplayer.com
Electrochemical Cells ppt download Which Energy Conversion Occurs In A Voltaic Cell The anode is an electrode where oxidation. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. The important parts of a voltaic cell: In a galvanic (voltaic) cell, the. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous. Which Energy Conversion Occurs In A Voltaic Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Which Energy Conversion Occurs In A Voltaic Cell The anode is an electrode where oxidation. A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. Chemical energy to electrical energy. Which energy conversions occurs in a operating voltaic cell. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. In a. Which Energy Conversion Occurs In A Voltaic Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Which Energy Conversion Occurs In A Voltaic Cell The important parts of a voltaic cell: An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. The anode is an electrode where oxidation. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Which energy conversions occurs in. Which Energy Conversion Occurs In A Voltaic Cell.
From www.youtube.com
9.4.1 Explain how a redox reaction is used to produce electricity in a Which Energy Conversion Occurs In A Voltaic Cell The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. The anode is an electrode where oxidation. Chemical energy to electrical energy. A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. Which energy conversions occurs in a operating voltaic cell. A voltaic. Which Energy Conversion Occurs In A Voltaic Cell.
From www.mrsolar.com
What Is Solar Energy? Which Energy Conversion Occurs In A Voltaic Cell In a galvanic (voltaic) cell, the. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. Chemical energy to electrical energy. Which energy conversions occurs in a operating voltaic cell. The galvanic cell, or. Which Energy Conversion Occurs In A Voltaic Cell.
From www.electronicsandyou.com
PV Cell Working Principle How Solar Photovoltaic Cells Work Which Energy Conversion Occurs In A Voltaic Cell In a galvanic (voltaic) cell, the. Which energy conversions occurs in a operating voltaic cell. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. An electrochemical cell. Which Energy Conversion Occurs In A Voltaic Cell.
From hindiarise.com
भारत में सौर ऊर्जा और सौर ऊर्जा पार्क (Solar Energy & Solar Power Parks Which Energy Conversion Occurs In A Voltaic Cell The anode is an electrode where oxidation. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. In a galvanic (voltaic) cell, the. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A voltaic cell (also known as. Which Energy Conversion Occurs In A Voltaic Cell.
From slideplayer.com
OXIDATION AND REDUCTION ppt download Which Energy Conversion Occurs In A Voltaic Cell The important parts of a voltaic cell: Chemical energy to electrical energy. In a galvanic (voltaic) cell, the. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. A. Which Energy Conversion Occurs In A Voltaic Cell.
From www.pinterest.pt
photovoltaic panels diagram Google Search Solar panels, Solar, How Which Energy Conversion Occurs In A Voltaic Cell In a galvanic (voltaic) cell, the. The anode is an electrode where oxidation. Which energy conversions occurs in a operating voltaic cell. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. A. Which Energy Conversion Occurs In A Voltaic Cell.
From slideplayer.com
Voltaic Cells Aim To identify the components and explain the functions Which Energy Conversion Occurs In A Voltaic Cell The anode is an electrode where oxidation. A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to. Which Energy Conversion Occurs In A Voltaic Cell.
From slideplayer.com
BATTERIES. Battery Definition A battery is a storage device used Which Energy Conversion Occurs In A Voltaic Cell In a galvanic (voltaic) cell, the. Chemical energy to electrical energy. A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. The important parts of a voltaic cell: An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. Which energy conversions occurs in a. Which Energy Conversion Occurs In A Voltaic Cell.
From www.slideserve.com
PPT Energy Flow in the Cell PowerPoint Presentation, free download Which Energy Conversion Occurs In A Voltaic Cell The anode is an electrode where oxidation. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. Which energy conversions occurs in a operating voltaic cell. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A voltaic cell. Which Energy Conversion Occurs In A Voltaic Cell.
From brainly.com
Which energy conversion occurs in the cells of an animal, such as the Which Energy Conversion Occurs In A Voltaic Cell Which energy conversions occurs in a operating voltaic cell. A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. Chemical energy to electrical energy. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A voltaic cell (also known as a galvanic cell). Which Energy Conversion Occurs In A Voltaic Cell.
From chem.libretexts.org
Voltaic Cells Chemistry LibreTexts Which Energy Conversion Occurs In A Voltaic Cell In a galvanic (voltaic) cell, the. The anode is an electrode where oxidation. A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. The important parts of a voltaic cell: A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Chemical energy to. Which Energy Conversion Occurs In A Voltaic Cell.
From www.youtube.com
GCSE CHEMISTRY ELECTRO CHEMISTRY LESSON 11 simple voltaic cell Which Energy Conversion Occurs In A Voltaic Cell The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. In a galvanic (voltaic) cell, the. Which energy conversions occurs in a operating voltaic cell. A voltaic cell (also. Which Energy Conversion Occurs In A Voltaic Cell.
From saylordotorg.github.io
Standard Potentials Which Energy Conversion Occurs In A Voltaic Cell Chemical energy to electrical energy. In a galvanic (voltaic) cell, the. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts. Which Energy Conversion Occurs In A Voltaic Cell.
From www.chegg.com
Solved Which two possible forms of energy conversion occurs Which Energy Conversion Occurs In A Voltaic Cell The anode is an electrode where oxidation. A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. Chemical energy to electrical energy. A voltaic cell (also known as a galvanic cell) is an electrochemical. Which Energy Conversion Occurs In A Voltaic Cell.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Which Energy Conversion Occurs In A Voltaic Cell A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The anode is an electrode where oxidation. A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy. Which Energy Conversion Occurs In A Voltaic Cell.
From www.sciencefacts.net
Energy Transformation (Conversion) Definition and Examples Which Energy Conversion Occurs In A Voltaic Cell A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. In a galvanic (voltaic) cell, the. Which energy conversions occurs in a operating voltaic cell. A voltaic cell (also known as a galvanic. Which Energy Conversion Occurs In A Voltaic Cell.
From www.transformationtutoring.com
Regents Chemistry Exam Multiple Choice Practice With Solutions Which Energy Conversion Occurs In A Voltaic Cell The important parts of a voltaic cell: Chemical energy to electrical energy. Which energy conversions occurs in a operating voltaic cell. In a galvanic (voltaic) cell, the. The anode is an electrode where oxidation. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A voltaic cell is an. Which Energy Conversion Occurs In A Voltaic Cell.
From www.youngwonks.com
Simply Explained The Flow of Electricity Which Energy Conversion Occurs In A Voltaic Cell Which energy conversions occurs in a operating voltaic cell. The important parts of a voltaic cell: Chemical energy to electrical energy. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. In a galvanic (voltaic) cell, the. The galvanic cell, or called voltaic cell, is an electrochemical cell that. Which Energy Conversion Occurs In A Voltaic Cell.
From energyeducation.ca
Photovoltaic effect Energy Education Which Energy Conversion Occurs In A Voltaic Cell The important parts of a voltaic cell: The anode is an electrode where oxidation. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A voltaic cell is an electrochemical cell that uses a chemical reaction to produce electrical energy. Chemical energy to electrical energy. An electrochemical cell can. Which Energy Conversion Occurs In A Voltaic Cell.
From www.researchgate.net
OER in electrochemical energy conversion and storage devices. a Which Energy Conversion Occurs In A Voltaic Cell Which energy conversions occurs in a operating voltaic cell. The anode is an electrode where oxidation. Chemical energy to electrical energy. In a galvanic (voltaic) cell, the. The important parts of a voltaic cell: An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. A voltaic cell is an electrochemical. Which Energy Conversion Occurs In A Voltaic Cell.