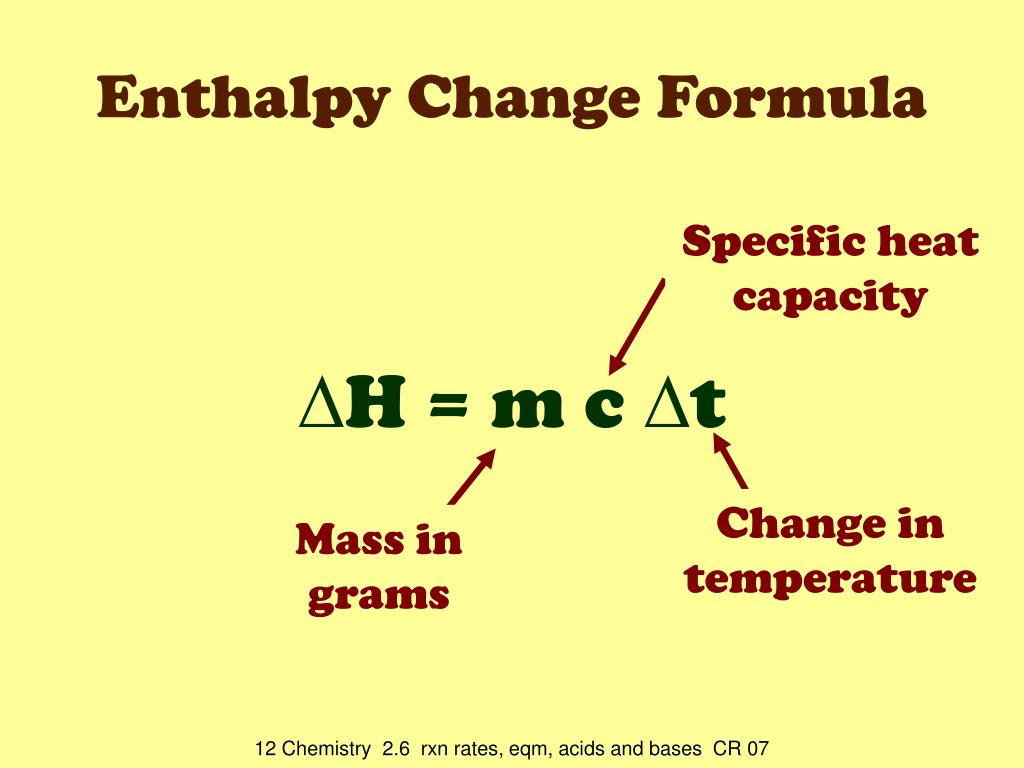
Calorimeter Formula . calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. — calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. — calorimetry formula: calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during some. Learn how to use the. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a fascinating branch of thermodynamics that enables us to. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature.
from
calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during some. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn how to use the. Calorimetry is a fascinating branch of thermodynamics that enables us to. — calorimetry formula: — calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two.
Calorimeter Formula calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during some. — calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. — calorimetry formula: Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn how to use the. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a fascinating branch of thermodynamics that enables us to. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during some.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimeter Formula Learn how to use the. Calorimetry is a fascinating branch of thermodynamics that enables us to. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt. Calorimeter Formula.
From
Calorimeter Formula — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during some. Calorimetry is the measurement of the transfer of heat into or out. Calorimeter Formula.
From www.britannica.com
Bomb calorimeter measurement device Britannica Calorimeter Formula q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. — calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. calorimetry is the part of chemistry which is about the study of the quantity. Calorimeter Formula.
From
Calorimeter Formula Learn how to use the. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron). Calorimeter Formula.
From
Calorimeter Formula calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Learn how to use the. — calorimetry formula: Calorimetry is. Calorimeter Formula.
From
Calorimeter Formula — calorimetry formula: — calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn how to use the. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure'). Calorimeter Formula.
From chemistrytalk.org
Calorimetry ChemTalk Calorimeter Formula Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. — calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with. Calorimeter Formula.
From
Calorimeter Formula calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. — calorimetry formula: in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimetry is the part of chemistry which is about the study of the quantity of heat which is. Calorimeter Formula.
From
Calorimeter Formula q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. — a calorimeter is a device used to measure the amount of heat. Calorimeter Formula.
From
Calorimeter Formula — calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. — calorimetry formula: calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during some. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον. Calorimeter Formula.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID1792465 Calorimeter Formula calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during some. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. — a calorimeter. Calorimeter Formula.
From
Calorimeter Formula q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. Learn how to use the. Calorimetry is the measurement of the transfer of heat. Calorimeter Formula.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Formula — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. Learn how to use the. — calorimetry formula: in chemistry and thermodynamics, calorimetry (from latin calor. Calorimeter Formula.
From
Calorimeter Formula q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimetry is the part of chemistry which is about the study of the. Calorimeter Formula.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter Formula — calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during some. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the.. Calorimeter Formula.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Calorimeter Formula Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. Calorimetry is a fascinating branch of thermodynamics that enables us to. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the part of chemistry. Calorimeter Formula.
From
Calorimeter Formula Learn how to use the. calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during some. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. — calorimetry formula: q = mcδt, where q is. Calorimeter Formula.
From
Calorimeter Formula — calorimetry formula: Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure'). Calorimeter Formula.
From
Calorimeter Formula Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. — a calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimeter Formula.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Formula Calorimetry is a fascinating branch of thermodynamics that enables us to. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. — calorimetry formula: in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. — a calorimeter is a device. Calorimeter Formula.
From
Calorimeter Formula — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a fascinating branch of thermodynamics that enables us to. Learn how to use the. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. q. Calorimeter Formula.
From
Calorimeter Formula — calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Learn how to use the. — calorimetry formula: in chemistry and thermodynamics, calorimetry. Calorimeter Formula.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimeter Formula calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during some. Calorimetry is a fascinating branch of thermodynamics that enables us to. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. . Calorimeter Formula.
From
Calorimeter Formula Learn how to use the. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the part. Calorimeter Formula.
From saylordotorg.github.io
Calorimetry Calorimeter Formula — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. — calorimetry formula: calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or. Calorimeter Formula.
From
Calorimeter Formula in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. Calorimetry is a fascinating branch of thermodynamics that enables us to. Learn how to use the. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. — calculate heat, temperature change,. Calorimeter Formula.
From
Calorimeter Formula — calorimetry formula: — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. — calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. calorimetry is the part of chemistry which is about the study of the quantity of heat which is. Calorimeter Formula.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6655927 Calorimeter Formula in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. — calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. Learn how to use the. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. q. Calorimeter Formula.
From
Calorimeter Formula — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a fascinating branch of thermodynamics that enables us to. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. — calorimetry formula: calorimetry is. Calorimeter Formula.
From
Calorimeter Formula calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. — calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. Learn how to use the. Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction. Calorimeter Formula.
From
Calorimeter Formula calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during some. Learn how to use the. — calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. Calorimetry is a fascinating branch of thermodynamics that enables us to. . Calorimeter Formula.
From
Calorimeter Formula Calorimetry is a fascinating branch of thermodynamics that enables us to. — calorimetry formula: Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. Learn how to use the. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimetry. Calorimeter Formula.
From
Calorimeter Formula — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. — calculate heat, temperature change, and specific heat after. Calorimeter Formula.
From
Calorimeter Formula Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. — calorimetry formula: — a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron). Calorimeter Formula.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Formula Calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. — calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. calorimetry is a field of. Calorimeter Formula.