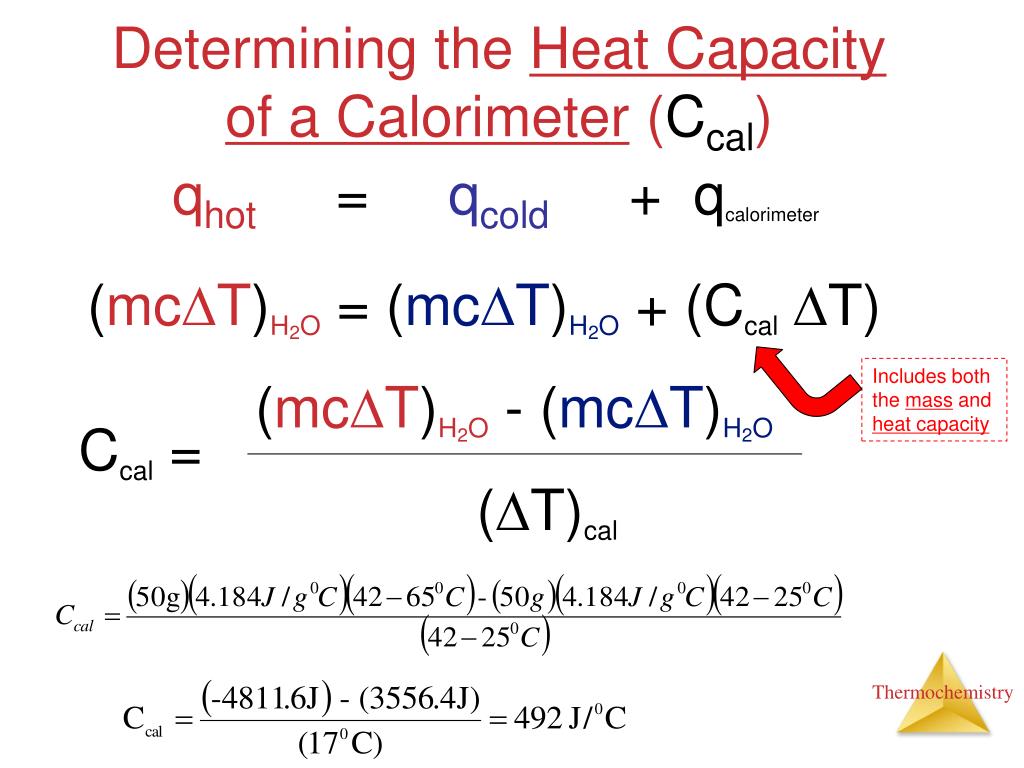
How To Find The Heat Capacity Of A Bomb Calorimeter . then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. This will be obtained by. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. C v is the heat capacity of the. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of.
from www.slideserve.com
C v is the heat capacity of the. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. This will be obtained by. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt.
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download
How To Find The Heat Capacity Of A Bomb Calorimeter then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. This will be obtained by. C v is the heat capacity of the. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net.
From 2012books.lardbucket.org
Calorimetry How To Find The Heat Capacity Of A Bomb Calorimeter prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. C v is the heat capacity of the. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts How To Find The Heat Capacity Of A Bomb Calorimeter the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. constant volume calorimetry, also know as bomb. How To Find The Heat Capacity Of A Bomb Calorimeter.
From socratic.org
When 0.602 g of biphenyl (C12H10) undergoes combustion in a bomb How To Find The Heat Capacity Of A Bomb Calorimeter This will be obtained by. prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. C v is the heat capacity of the. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. constant volume calorimetry, also know as bomb calorimetry, is. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube How To Find The Heat Capacity Of A Bomb Calorimeter prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and.. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation, free download ID3206969 How To Find The Heat Capacity Of A Bomb Calorimeter then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. This will be obtained by. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. prior to any measurement for a. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube How To Find The Heat Capacity Of A Bomb Calorimeter This will be obtained by. C v is the heat capacity of the. prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. then. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube How To Find The Heat Capacity Of A Bomb Calorimeter prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. C v is the heat capacity of the. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.numerade.com
SOLVED A bomb calorimeter or a constant volume calorimeler; is a How To Find The Heat Capacity Of A Bomb Calorimeter then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. C v is the heat capacity of the. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. the bomb is. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download How To Find The Heat Capacity Of A Bomb Calorimeter This will be obtained by. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. prior to any measurement for a. How To Find The Heat Capacity Of A Bomb Calorimeter.
From coursestar.com
Mastery Bomb Calorimetry Calculate Heat Capacity of Calorimeter How To Find The Heat Capacity Of A Bomb Calorimeter the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. This will be obtained by. constant volume calorimetry, also know as bomb calorimetry, is used. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation ID3206969 How To Find The Heat Capacity Of A Bomb Calorimeter then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. C v is the heat capacity of the. This will be obtained. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download How To Find The Heat Capacity Of A Bomb Calorimeter heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. C v is the heat capacity of the. prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. This will be obtained by. the. How To Find The Heat Capacity Of A Bomb Calorimeter.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson How To Find The Heat Capacity Of A Bomb Calorimeter C v is the heat capacity of the. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. the bomb is placed in a. How To Find The Heat Capacity Of A Bomb Calorimeter.
From people.chem.umass.edu
Untitled Document [people.chem.umass.edu] How To Find The Heat Capacity Of A Bomb Calorimeter heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. the bomb is placed in a water container and by measuring. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec How To Find The Heat Capacity Of A Bomb Calorimeter heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. C v is the heat capacity of the. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. constant volume calorimetry,. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec How To Find The Heat Capacity Of A Bomb Calorimeter C v is the heat capacity of the. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. This. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.slideshare.net
Thermochemistry by rkansal15’s How To Find The Heat Capacity Of A Bomb Calorimeter the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. C v is the heat capacity of the. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. prior to any measurement. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation How To Find The Heat Capacity Of A Bomb Calorimeter the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download How To Find The Heat Capacity Of A Bomb Calorimeter heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. C v is the heat capacity of the. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. . How To Find The Heat Capacity Of A Bomb Calorimeter.
From shaunmwilliams.com
Chapter 6 Presentation How To Find The Heat Capacity Of A Bomb Calorimeter C v is the heat capacity of the. prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. then use equation \ref{12.3.1} to determine. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.slideserve.com
PPT Introduction to Thermochemistry PowerPoint Presentation, free How To Find The Heat Capacity Of A Bomb Calorimeter then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. C v is the heat capacity of the. prior to any. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.chegg.com
Solved Calculate the heat capacity of the bomb calorimeter How To Find The Heat Capacity Of A Bomb Calorimeter C v is the heat capacity of the. prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. heat capacity of the calorimeter system can be determined, allowing for. How To Find The Heat Capacity Of A Bomb Calorimeter.
From exobdylqi.blob.core.windows.net
Bomb Calorimeter What Does It Do at Joann Guarino blog How To Find The Heat Capacity Of A Bomb Calorimeter prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. the bomb is placed in a water container and by measuring its temperature change. How To Find The Heat Capacity Of A Bomb Calorimeter.
From byjus.com
What is bomb calorimeter? How To Find The Heat Capacity Of A Bomb Calorimeter This will be obtained by. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb). How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.britannica.com
Bomb calorimeter measurement device Britannica How To Find The Heat Capacity Of A Bomb Calorimeter heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. prior to any measurement for a sample in the calorimeter, its. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.labster.com
5 Ways to Make Calorimetry and the Bomb Calorimeter More Approachable How To Find The Heat Capacity Of A Bomb Calorimeter then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. C v is the heat capacity of the. This will be obtained by. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. the bomb is. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube How To Find The Heat Capacity Of A Bomb Calorimeter C v is the heat capacity of the. prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. then use equation \ref{12.3.1} to determine the heat capacity of. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download How To Find The Heat Capacity Of A Bomb Calorimeter This will be obtained by. prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. heat capacity of the calorimeter system can be determined, allowing for the calculation of. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube How To Find The Heat Capacity Of A Bomb Calorimeter constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii How To Find The Heat Capacity Of A Bomb Calorimeter heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. prior to any measurement for a sample. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.thoughtco.com
Calorimeter Definition in Chemistry How To Find The Heat Capacity Of A Bomb Calorimeter constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. prior to any measurement for a sample in the calorimeter, its heat. How To Find The Heat Capacity Of A Bomb Calorimeter.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter How To Find The Heat Capacity Of A Bomb Calorimeter C v is the heat capacity of the. This will be obtained by. prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion of a sample of known mass by the net. the. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.shutterstock.com
Bomb Calorimeter Vector Illustration Labeled Educational Stock Vector How To Find The Heat Capacity Of A Bomb Calorimeter the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.bartleby.com
Answered A bomb calorimeter, or a constant… bartleby How To Find The Heat Capacity Of A Bomb Calorimeter prior to any measurement for a sample in the calorimeter, its heat capacity must be determined. the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of combustion. How To Find The Heat Capacity Of A Bomb Calorimeter.
From www.numerade.com
SOLVEDUse the References to access important values if needed for this How To Find The Heat Capacity Of A Bomb Calorimeter constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. C v is the heat capacity of the. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. prior to any measurement for a sample in. How To Find The Heat Capacity Of A Bomb Calorimeter.