Iron Neutrons Electron . The iron atom has four stable isotopes. Neutrons are neutral particles with no charge. Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. Therefore, an iron atom has thirty neutrons. The mass of a proton or a neutron is about 1836 times greater than the mass of an. Protons and neutrons are found in the nucleus,. The number of neutrons depends on the isotope of the element. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Iron has four stable isotopes: Neutrons are relatively heavy particles. 54fe (5.845% of natural iron), 56fe (91.754%), 57fe (2.119%) and 58fe (0.282%). In this article, i have. Iron typically has 30 neutrons in its most common isotope. Atoms are made up of three types of subatomic particles: Neutrons have approximately the same mass as protons but no charge.
from www.psi.ch
Neutrons have approximately the same mass as protons but no charge. In this article, i have. The number of neutrons depends on the isotope of the element. 54fe (5.845% of natural iron), 56fe (91.754%), 57fe (2.119%) and 58fe (0.282%). Therefore, an iron atom has thirty neutrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Neutrons are relatively heavy particles. Atoms are made up of three types of subatomic particles: The iron atom has four stable isotopes. Iron has four stable isotopes:
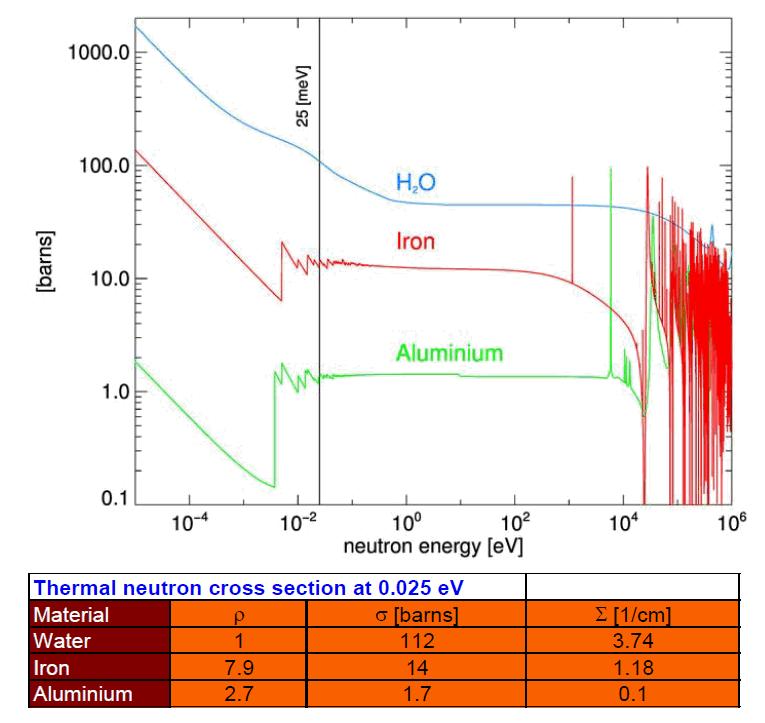
Neutron Matter Interaction Paul Scherrer Institut (PSI)
Iron Neutrons Electron Atoms are made up of three types of subatomic particles: Neutrons are neutral particles with no charge. The iron atom has four stable isotopes. Atoms are made up of three types of subatomic particles: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. Therefore, an iron atom has thirty neutrons. Iron has four stable isotopes: In this article, i have. Protons and neutrons are found in the nucleus,. 54fe (5.845% of natural iron), 56fe (91.754%), 57fe (2.119%) and 58fe (0.282%). Iron typically has 30 neutrons in its most common isotope. Neutrons are relatively heavy particles. The mass of a proton or a neutron is about 1836 times greater than the mass of an. Neutrons have approximately the same mass as protons but no charge. The number of neutrons depends on the isotope of the element.
From makeup.vidalondon.net
What Is The Atomic Makeup Of Iron Makeup Vidalondon Iron Neutrons Electron Neutrons are relatively heavy particles. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. The number of neutrons depends on the isotope of the element. Neutrons have approximately the same mass as protons but no charge. Protons and neutrons are found in the nucleus,. In this article, i have. Atoms are made up of three types of subatomic particles: Protons. Iron Neutrons Electron.
From www.sciencephoto.com
Iron, atomic structure Stock Image C018/3707 Science Photo Library Iron Neutrons Electron Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. The number of neutrons depends on the isotope of the element. The mass of a proton or a neutron is about 1836 times greater than the mass of an. Neutrons have approximately the same mass as protons but no charge. Atoms are made up. Iron Neutrons Electron.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Iron (Fe Iron Neutrons Electron Neutrons are neutral particles with no charge. The iron atom has four stable isotopes. In this article, i have. Atoms are made up of three types of subatomic particles: The mass of a proton or a neutron is about 1836 times greater than the mass of an. The number of neutrons depends on the isotope of the element. Neutrons are. Iron Neutrons Electron.
From elchoroukhost.net
Iron Periodic Table Protons Neutrons And Electrons Elcho Table Iron Neutrons Electron Neutrons are neutral particles with no charge. Iron has four stable isotopes: The iron atom has four stable isotopes. Protons and neutrons are found in the nucleus,. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. The mass of a proton or a neutron is about 1836 times greater than the mass of an. Iron typically has 30 neutrons in. Iron Neutrons Electron.
From www.bigstockphoto.com
3d Render Atom Structure Iron Image & Photo Bigstock Iron Neutrons Electron Therefore, an iron atom has thirty neutrons. In this article, i have. 54fe (5.845% of natural iron), 56fe (91.754%), 57fe (2.119%) and 58fe (0.282%). The iron atom has four stable isotopes. Protons and neutrons are found in the nucleus,. Neutrons are neutral particles with no charge. The number of neutrons depends on the isotope of the element. Sources, facts, uses,. Iron Neutrons Electron.
From utedzz.blogspot.com
Periodic Table Numbers Of Neutrons Protons And Electrons Periodic Iron Neutrons Electron 54fe (5.845% of natural iron), 56fe (91.754%), 57fe (2.119%) and 58fe (0.282%). The iron atom has four stable isotopes. Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. Neutrons are relatively heavy particles. Neutrons are neutral particles with no charge. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Neutrons have approximately the. Iron Neutrons Electron.
From hxerdbrvt.blob.core.windows.net
Iron Protons Electrons And Neutrons at Sheryl Crawford blog Iron Neutrons Electron Neutrons are neutral particles with no charge. 54fe (5.845% of natural iron), 56fe (91.754%), 57fe (2.119%) and 58fe (0.282%). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. The mass of a proton or a neutron is about 1836 times greater than the mass of an. Neutrons have approximately the same mass as protons but no charge. Therefore, an iron. Iron Neutrons Electron.
From www.youtube.com
Atomic Structure Protons, Electrons & Neutrons YouTube Iron Neutrons Electron Neutrons are relatively heavy particles. Neutrons have approximately the same mass as protons but no charge. Protons and neutrons are found in the nucleus,. 54fe (5.845% of natural iron), 56fe (91.754%), 57fe (2.119%) and 58fe (0.282%). Atoms are made up of three types of subatomic particles: The iron atom has four stable isotopes. Iron typically has 30 neutrons in its. Iron Neutrons Electron.
From reviewhomedecor.co
Iron Periodic Table Protons Neutrons Electrons Review Home Decor Iron Neutrons Electron The number of neutrons depends on the isotope of the element. Iron has four stable isotopes: Iron typically has 30 neutrons in its most common isotope. Atoms are made up of three types of subatomic particles: Neutrons are relatively heavy particles. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Protons and neutrons are found in the nucleus,. Therefore, an. Iron Neutrons Electron.
From www.numerade.com
SOLVED Determine the number of (a) electrons, (b) protons, and (c Iron Neutrons Electron Iron has four stable isotopes: Neutrons are neutral particles with no charge. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. In this article, i have. Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. The iron atom has four stable isotopes. The mass of a proton or a neutron is about 1836. Iron Neutrons Electron.
From reviewhomedecor.co
Iron Periodic Table Protons Neutrons Electrons Review Home Decor Iron Neutrons Electron Iron has four stable isotopes: The number of neutrons depends on the isotope of the element. In this article, i have. Iron typically has 30 neutrons in its most common isotope. Neutrons are neutral particles with no charge. Atoms are made up of three types of subatomic particles: Neutrons have approximately the same mass as protons but no charge. Neutrons. Iron Neutrons Electron.
From www.expii.com
Neutrons — Structure & Properties Expii Iron Neutrons Electron Iron typically has 30 neutrons in its most common isotope. Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. Neutrons are neutral particles with no charge. Neutrons have approximately the same mass as protons but no charge. The mass of a proton or a neutron is about 1836 times greater than the mass. Iron Neutrons Electron.
From general.chemistrysteps.com
Balancing Nuclear Reactions Chemistry Steps Iron Neutrons Electron Protons and neutrons are found in the nucleus,. In this article, i have. The mass of a proton or a neutron is about 1836 times greater than the mass of an. Neutrons are neutral particles with no charge. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Protons are relatively heavy particles with a charge of 1+ and a mass. Iron Neutrons Electron.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Iron Neutrons Electron Atoms are made up of three types of subatomic particles: Therefore, an iron atom has thirty neutrons. Neutrons have approximately the same mass as protons but no charge. The number of neutrons depends on the isotope of the element. The iron atom has four stable isotopes. Protons are relatively heavy particles with a charge of 1+ and a mass of. Iron Neutrons Electron.
From www.periodictableprintable.com
Iron Periodic Table Protons Neutrons And Electrons 2023 Periodic Iron Neutrons Electron Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. In this article, i have. Iron typically has 30 neutrons in its most common isotope. Protons and neutrons are found in the nucleus,. Neutrons are relatively heavy particles. Atoms are made up of three types of subatomic particles: Neutrons are neutral particles with no. Iron Neutrons Electron.
From www.sciencefacts.net
Neutron Definition, Characteristics, & Location with Example Iron Neutrons Electron Atoms are made up of three types of subatomic particles: Iron typically has 30 neutrons in its most common isotope. The number of neutrons depends on the isotope of the element. In this article, i have. Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. Neutrons are neutral particles with no charge. Neutrons. Iron Neutrons Electron.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Iron Neutrons Electron Neutrons are neutral particles with no charge. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Therefore, an iron atom has thirty neutrons. Iron typically has 30 neutrons in its most common isotope. In this article, i have. Iron has four stable isotopes: The iron atom has four stable isotopes. The mass of a proton or a neutron is about. Iron Neutrons Electron.
From www.numerade.com
SOLVED How many protons, neutrons, and electrons are present in an Iron Neutrons Electron Neutrons have approximately the same mass as protons but no charge. Therefore, an iron atom has thirty neutrons. Atoms are made up of three types of subatomic particles: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. 54fe (5.845% of natural iron), 56fe (91.754%), 57fe (2.119%) and 58fe (0.282%). Iron typically has 30 neutrons in its most common isotope. The. Iron Neutrons Electron.
From valenceelectrons.com
Iron(Fe) electron configuration and orbital diagram Iron Neutrons Electron Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. Protons and neutrons are found in the nucleus,. Iron has four stable isotopes: The number of neutrons depends on the isotope of the element. 54fe (5.845% of natural iron), 56fe (91.754%), 57fe (2.119%) and 58fe (0.282%). The mass of a proton or a neutron. Iron Neutrons Electron.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Iron Neutrons Electron 54fe (5.845% of natural iron), 56fe (91.754%), 57fe (2.119%) and 58fe (0.282%). Atoms are made up of three types of subatomic particles: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Iron typically has 30 neutrons in its most common isotope. Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. Neutrons have approximately. Iron Neutrons Electron.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Iron Neutrons Electron The mass of a proton or a neutron is about 1836 times greater than the mass of an. Protons and neutrons are found in the nucleus,. Iron typically has 30 neutrons in its most common isotope. Iron has four stable isotopes: The iron atom has four stable isotopes. Neutrons have approximately the same mass as protons but no charge. 54fe. Iron Neutrons Electron.
From www.numerade.com
SOLVED How many protons, neutrons, and electrons are in an iron(III Iron Neutrons Electron In this article, i have. The iron atom has four stable isotopes. Iron has four stable isotopes: Neutrons are relatively heavy particles. The mass of a proton or a neutron is about 1836 times greater than the mass of an. Iron typically has 30 neutrons in its most common isotope. Protons and neutrons are found in the nucleus,. Atoms are. Iron Neutrons Electron.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Iron Neutrons Electron The mass of a proton or a neutron is about 1836 times greater than the mass of an. 54fe (5.845% of natural iron), 56fe (91.754%), 57fe (2.119%) and 58fe (0.282%). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Protons and neutrons are found in the nucleus,. Protons are relatively heavy particles with a charge of 1+ and a mass. Iron Neutrons Electron.
From www.youtube.com
How to find Protons & Electrons for Fe2+ and Fe3+ (Iron II and III ions Iron Neutrons Electron Neutrons are relatively heavy particles. Neutrons are neutral particles with no charge. Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. The mass of a proton or a neutron is about 1836 times greater than the mass of an. Iron has four stable isotopes: Protons and neutrons are found in the nucleus,. In. Iron Neutrons Electron.
From www.youtube.com
Protons, neutrons, electrons iron55 006 YouTube Iron Neutrons Electron Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. Neutrons are neutral particles with no charge. Iron typically has 30 neutrons in its most common isotope. In this article, i have. Neutrons are relatively heavy particles. 54fe (5.845% of natural iron), 56fe (91.754%), 57fe (2.119%) and 58fe (0.282%). Atoms are made up of. Iron Neutrons Electron.
From www.psi.ch
Neutron Matter Interaction Paul Scherrer Institut (PSI) Iron Neutrons Electron In this article, i have. Iron has four stable isotopes: Therefore, an iron atom has thirty neutrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. The mass of a proton or a neutron is about 1836 times greater than the mass of an. Neutrons have approximately the same mass as protons but no charge. The iron atom has four. Iron Neutrons Electron.
From hxerdbrvt.blob.core.windows.net
Iron Protons Electrons And Neutrons at Sheryl Crawford blog Iron Neutrons Electron Neutrons have approximately the same mass as protons but no charge. The mass of a proton or a neutron is about 1836 times greater than the mass of an. Neutrons are neutral particles with no charge. Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. Atoms are made up of three types of. Iron Neutrons Electron.
From sciencenotes.org
Iron Facts Atomic Number 26 or Fe Iron Neutrons Electron Neutrons have approximately the same mass as protons but no charge. 54fe (5.845% of natural iron), 56fe (91.754%), 57fe (2.119%) and 58fe (0.282%). The number of neutrons depends on the isotope of the element. Neutrons are relatively heavy particles. In this article, i have. Neutrons are neutral particles with no charge. Therefore, an iron atom has thirty neutrons. Atoms are. Iron Neutrons Electron.
From valenceelectrons.com
How many protons, neutrons and electrons does iron have? Iron Neutrons Electron Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. The mass of a proton or a neutron is about 1836 times greater than the mass of an. Iron has four stable isotopes: Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. 54fe (5.845% of natural iron), 56fe (91.754%), 57fe (2.119%) and 58fe (0.282%).. Iron Neutrons Electron.
From material-properties.org
Iron Protons Neutrons Electrons Electron Configuration Iron Neutrons Electron Iron has four stable isotopes: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Atoms are made up of three types of subatomic particles: Neutrons have approximately the same mass as protons but no charge. In this article, i have. The iron atom has four stable isotopes. Therefore, an iron atom has thirty neutrons. Iron typically has 30 neutrons in. Iron Neutrons Electron.
From chemistry291.blogspot.com
【3 Steps】How Many Neutrons Does Iron(Fe) Have?Number of Neutrons in Iron Neutrons Electron Iron has four stable isotopes: Neutrons are neutral particles with no charge. The number of neutrons depends on the isotope of the element. The iron atom has four stable isotopes. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Neutrons are relatively heavy particles. Protons and neutrons are found in the nucleus,. Neutrons have approximately the same mass as protons. Iron Neutrons Electron.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Iron Neutrons Electron Neutrons are neutral particles with no charge. Neutrons have approximately the same mass as protons but no charge. Therefore, an iron atom has thirty neutrons. The iron atom has four stable isotopes. Iron typically has 30 neutrons in its most common isotope. Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. Iron has. Iron Neutrons Electron.
From sciencing.com
How to Find the Neutrons in the Periodic Table Sciencing Iron Neutrons Electron Neutrons have approximately the same mass as protons but no charge. Protons and neutrons are found in the nucleus,. The mass of a proton or a neutron is about 1836 times greater than the mass of an. Iron has four stable isotopes: Therefore, an iron atom has thirty neutrons. Neutrons are relatively heavy particles. Atoms are made up of three. Iron Neutrons Electron.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron Neutrons Electron Therefore, an iron atom has thirty neutrons. Neutrons are relatively heavy particles. The mass of a proton or a neutron is about 1836 times greater than the mass of an. The number of neutrons depends on the isotope of the element. Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. Neutrons are neutral. Iron Neutrons Electron.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron Neutrons Electron Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Neutrons are relatively heavy particles. The number of neutrons depends on the isotope of the element. Therefore, an iron atom has thirty neutrons. Iron typically has 30 neutrons in its most common isotope. Neutrons have approximately the same mass as protons but no charge. 54fe (5.845% of natural iron), 56fe (91.754%),. Iron Neutrons Electron.