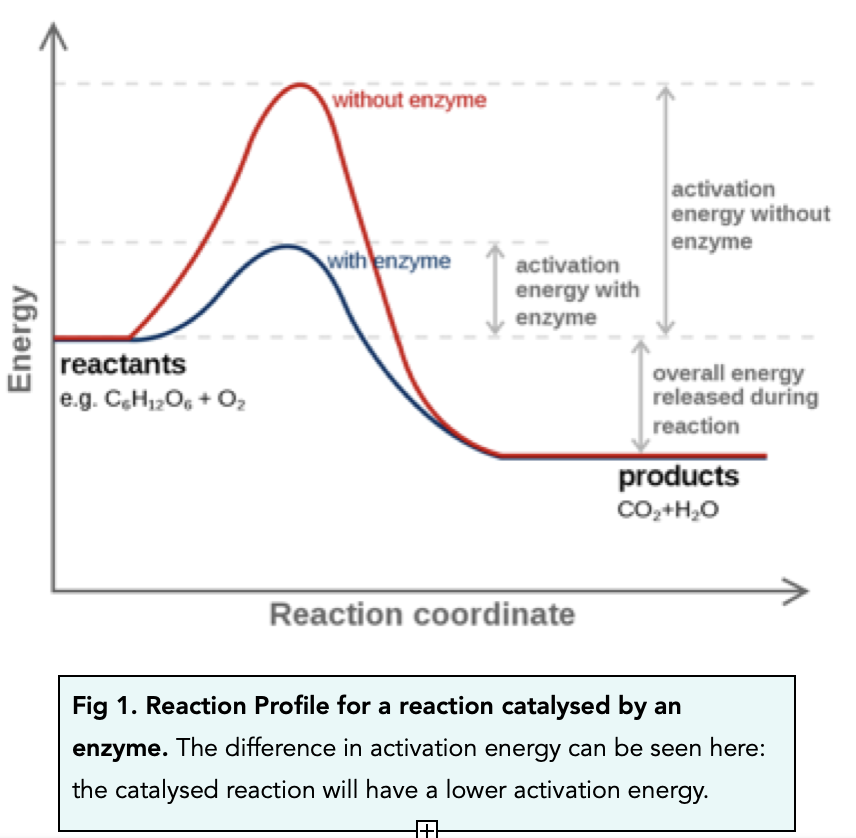
Catalyst Chemistry As Level . Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. They provide an alternative reaction route with a lower activation. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Catalysts increase reaction rates without getting used up. Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism of reactions. They do this by providing an alternative reaction pathway with a lower activation energy. Reverses the preparation of an ester from an alcohol and a carboxylic acid. A catalyst is a substance which speeds up the rate of a reaction without being chemically changed at the end. A catalyst is a substance which increases the rate of a reaction without being used up itself. A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower in activation.
from studymind.co.uk
A catalyst is a substance which increases the rate of a reaction without being used up itself. They do this by providing an alternative reaction pathway with a lower activation energy. They provide an alternative reaction route with a lower activation. Reverses the preparation of an ester from an alcohol and a carboxylic acid. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. A catalyst is a substance which speeds up the rate of a reaction without being chemically changed at the end. A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower in activation. Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism of reactions. Catalysts increase reaction rates without getting used up.
Catalysts (GCSE Chemistry) Study Mind
Catalyst Chemistry As Level A catalyst is a substance which increases the rate of a reaction without being used up itself. A catalyst is a substance which increases the rate of a reaction without being used up itself. A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower in activation. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. They do this by providing an alternative reaction pathway with a lower activation energy. Catalysts increase reaction rates without getting used up. They provide an alternative reaction route with a lower activation. Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism of reactions. Reverses the preparation of an ester from an alcohol and a carboxylic acid. A catalyst is a substance which speeds up the rate of a reaction without being chemically changed at the end.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记6.2.10 Heterogeneous Catalysis翰林国际教育 Catalyst Chemistry As Level A catalyst is a substance which increases the rate of a reaction without being used up itself. A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower in activation. Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism of reactions. They provide an alternative reaction route. Catalyst Chemistry As Level.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Chemistry As Level A catalyst is a substance which increases the rate of a reaction without being used up itself. Reverses the preparation of an ester from an alcohol and a carboxylic acid. Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism. Catalyst Chemistry As Level.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Chemistry As Level Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. They provide an alternative reaction route with a lower activation. Reverses the preparation of an ester from an alcohol and a carboxylic acid. A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway. Catalyst Chemistry As Level.
From philschatz.com
Catalysis · Chemistry Catalyst Chemistry As Level Reverses the preparation of an ester from an alcohol and a carboxylic acid. Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism of reactions. A catalyst is a substance which increases the rate of a reaction without being used up itself. A catalyst is a substance which speeds up the rate of a reaction without being. Catalyst Chemistry As Level.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalyst Chemistry As Level Catalysts increase reaction rates without getting used up. Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism of reactions. A catalyst is a substance which increases the rate of a reaction without being used up itself. They provide an alternative reaction route with a lower activation. Use our revision notes to understand catalytic converters for your. Catalyst Chemistry As Level.
From www.youtube.com
What are catalysts Chemistry for All The Fuse School YouTube Catalyst Chemistry As Level Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. A catalyst is a substance which speeds up the rate of a reaction without being chemically changed at the end. A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower. Catalyst Chemistry As Level.
From www.researchgate.net
Commonly Used Types of Catalysts and Their Range of Use Download Catalyst Chemistry As Level Reverses the preparation of an ester from an alcohol and a carboxylic acid. A catalyst is a substance which speeds up the rate of a reaction without being chemically changed at the end. Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism of reactions. Catalysts increase reaction rates without getting used up. Use our revision notes. Catalyst Chemistry As Level.
From www.savemyexams.com
Catalysts AQA GCSE Chemistry Revision Notes 2018 Catalyst Chemistry As Level They provide an alternative reaction route with a lower activation. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Reverses the preparation of an ester from an alcohol and a carboxylic acid. Catalysts increase reaction rates without getting used up. Catalysis is a cornerstone concept in chemistry, influencing both. Catalyst Chemistry As Level.
From 2012books.lardbucket.org
Catalysis Catalyst Chemistry As Level A catalyst is a substance which increases the rate of a reaction without being used up itself. Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism of reactions. Catalysts increase reaction rates without getting used up. Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. A catalyst increases. Catalyst Chemistry As Level.
From www.nagwa.com
Lesson Catalysts Nagwa Catalyst Chemistry As Level A catalyst is a substance which speeds up the rate of a reaction without being chemically changed at the end. They do this by providing an alternative reaction pathway with a lower activation energy. Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism of reactions. A catalyst is a substance which increases the rate of a. Catalyst Chemistry As Level.
From www.slideserve.com
PPT 23.5 Features of homogeneous catalysis PowerPoint Presentation Catalyst Chemistry As Level Catalysts increase reaction rates without getting used up. Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. Reverses the preparation of an ester from an alcohol and a carboxylic acid. A catalyst is a substance which increases the rate of a reaction without being used up itself. Use our revision notes to. Catalyst Chemistry As Level.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Chemistry As Level A catalyst is a substance which increases the rate of a reaction without being used up itself. Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism of reactions. Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. Reverses the preparation of an ester from an alcohol and a. Catalyst Chemistry As Level.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent Catalyst Chemistry As Level Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. They do this by providing an alternative reaction pathway with a lower activation energy. Catalysts increase reaction rates without getting used up. A catalyst is a substance which speeds up the rate of a reaction without being chemically changed at the end. Reverses. Catalyst Chemistry As Level.
From www.catalystseurope.org
What are catalysts? Catalyst Chemistry As Level Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism of reactions. Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. They do this by providing an alternative reaction. Catalyst Chemistry As Level.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Chemistry As Level Reverses the preparation of an ester from an alcohol and a carboxylic acid. Catalysts increase reaction rates without getting used up. Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. A catalyst is a substance which speeds up the rate of a reaction without being chemically changed at the end. Use our. Catalyst Chemistry As Level.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Chemistry As Level A catalyst is a substance which increases the rate of a reaction without being used up itself. A catalyst is a substance which speeds up the rate of a reaction without being chemically changed at the end. They provide an alternative reaction route with a lower activation. Revision notes for the cie as chemistry syllabus, written by the chemistry experts. Catalyst Chemistry As Level.
From www.chemistrystudent.com
Boltzmann Distribution Curves (ALevel) ChemistryStudent Catalyst Chemistry As Level A catalyst is a substance which increases the rate of a reaction without being used up itself. Catalysts increase reaction rates without getting used up. They provide an alternative reaction route with a lower activation. A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower in activation. They do this. Catalyst Chemistry As Level.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst Chemistry As Level A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower in activation. They do this by providing an alternative reaction pathway with a lower activation energy. A catalyst is a substance which increases the rate of a reaction without being used up itself. A catalyst is a substance which speeds. Catalyst Chemistry As Level.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Chemistry As Level A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower in activation. Catalysts increase reaction rates without getting used up. A catalyst is a substance which speeds up the rate of a reaction without being chemically changed at the end. Reverses the preparation of an ester from an alcohol and. Catalyst Chemistry As Level.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Catalyst Chemistry As Level A catalyst is a substance which speeds up the rate of a reaction without being chemically changed at the end. Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. They provide an. Catalyst Chemistry As Level.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Chemistry As Level A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower in activation. Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism of reactions. Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. Catalysts increase reaction rates without getting. Catalyst Chemistry As Level.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Chemistry As Level Reverses the preparation of an ester from an alcohol and a carboxylic acid. They do this by providing an alternative reaction pathway with a lower activation energy. A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower in activation. A catalyst is a substance which increases the rate of a. Catalyst Chemistry As Level.
From www.chemistrystudent.com
Catalysts (ALevel) ChemistryStudent Catalyst Chemistry As Level A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower in activation. They do this by providing an alternative reaction pathway with a lower activation energy. Reverses the preparation of an ester from an alcohol and a carboxylic acid. Use our revision notes to understand catalytic converters for your a. Catalyst Chemistry As Level.
From www.slideshare.net
Catalyst Catalyst Chemistry As Level Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. They do this by providing an alternative reaction pathway with a lower activation energy. A catalyst is a substance which increases the rate. Catalyst Chemistry As Level.
From www.researchgate.net
2. List of Catalysts Tested and Corresponding Labels Download Table Catalyst Chemistry As Level They provide an alternative reaction route with a lower activation. A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower in activation. Catalysts increase reaction rates without getting used up. They do this by providing an alternative reaction pathway with a lower activation energy. A catalyst is a substance which. Catalyst Chemistry As Level.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent Catalyst Chemistry As Level Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism of reactions. Reverses the preparation of an ester from an alcohol and a carboxylic acid. Catalysts increase reaction rates without getting used up. Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. A catalyst is a substance which speeds. Catalyst Chemistry As Level.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalyst Chemistry As Level A catalyst is a substance which increases the rate of a reaction without being used up itself. A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower in activation. They provide an alternative reaction route with a lower activation. Catalysts increase reaction rates without getting used up. Reverses the preparation. Catalyst Chemistry As Level.
From www.savemyexams.com
Types of Catalyst CIE A Level Chemistry Revision Notes 2025 Catalyst Chemistry As Level Reverses the preparation of an ester from an alcohol and a carboxylic acid. A catalyst is a substance which increases the rate of a reaction without being used up itself. They do this by providing an alternative reaction pathway with a lower activation energy. Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism of reactions. A. Catalyst Chemistry As Level.
From www.chemistrystudent.com
Catalysts (ALevel) ChemistryStudent Catalyst Chemistry As Level A catalyst is a substance which speeds up the rate of a reaction without being chemically changed at the end. A catalyst is a substance which increases the rate of a reaction without being used up itself. They provide an alternative reaction route with a lower activation. Revision notes for the cie as chemistry syllabus, written by the chemistry experts. Catalyst Chemistry As Level.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalyst Chemistry As Level A catalyst is a substance which increases the rate of a reaction without being used up itself. They provide an alternative reaction route with a lower activation. Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. Reverses the preparation of an ester from an alcohol and a carboxylic acid. Catalysts increase reaction. Catalyst Chemistry As Level.
From www.savemyexams.co.uk
Catalysts (1.7.6) AQA A Level Chemistry Revision Notes 2017 Save My Catalyst Chemistry As Level Reverses the preparation of an ester from an alcohol and a carboxylic acid. They do this by providing an alternative reaction pathway with a lower activation energy. A catalyst increases the rate of a reaction by providing the reactants with an alternative reaction pathway which is lower in activation. Revision notes for the cie as chemistry syllabus, written by the. Catalyst Chemistry As Level.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent Catalyst Chemistry As Level A catalyst is a substance which increases the rate of a reaction without being used up itself. They do this by providing an alternative reaction pathway with a lower activation energy. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Catalysis is a cornerstone concept in chemistry, influencing both. Catalyst Chemistry As Level.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Catalyst Chemistry As Level They provide an alternative reaction route with a lower activation. A catalyst is a substance which increases the rate of a reaction without being used up itself. They do this by providing an alternative reaction pathway with a lower activation energy. Catalysts increase reaction rates without getting used up. Catalysis is a cornerstone concept in chemistry, influencing both the rate. Catalyst Chemistry As Level.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID4503154 Catalyst Chemistry As Level They provide an alternative reaction route with a lower activation. Revision notes for the cie as chemistry syllabus, written by the chemistry experts at save my exams. Use our revision notes to understand catalytic converters for your a level chemistry course and their role in reducing pollution. Catalysts increase reaction rates without getting used up. A catalyst increases the rate. Catalyst Chemistry As Level.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalyst Chemistry As Level A catalyst is a substance which increases the rate of a reaction without being used up itself. They do this by providing an alternative reaction pathway with a lower activation energy. Catalysis is a cornerstone concept in chemistry, influencing both the rate and mechanism of reactions. A catalyst increases the rate of a reaction by providing the reactants with an. Catalyst Chemistry As Level.