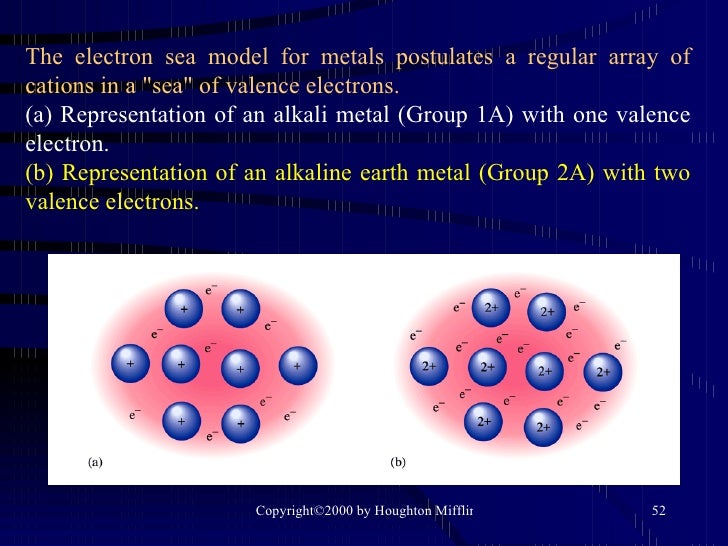
Iron Electron Sea Model . Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons (the blue dots). The sea of electrons surrounding the. A simplified model to describe metallic bonding has been developed by paul drüde. The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a mobile 'sea' of electrons. In contrast to electrons that participate in both ionic and covalent bonds, electrons that participate in metallic bonds delocalize, forming a sea of electrons around the positive. This electron sea model accounts for most physical. The sea of electrons model. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. Metallic bonds are formed between two metal atoms.
from aawesseaa.blogspot.com
The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons (the blue dots). Metallic bonds are formed between two metal atoms. A simplified model to describe metallic bonding has been developed by paul drüde. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. This electron sea model accounts for most physical. The sea of electrons surrounding the. The sea of electrons model. The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a mobile 'sea' of electrons. In contrast to electrons that participate in both ionic and covalent bonds, electrons that participate in metallic bonds delocalize, forming a sea of electrons around the positive.
Electron Sea Model Definition
Iron Electron Sea Model The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. In contrast to electrons that participate in both ionic and covalent bonds, electrons that participate in metallic bonds delocalize, forming a sea of electrons around the positive. A simplified model to describe metallic bonding has been developed by paul drüde. Metallic bonds are formed between two metal atoms. The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons (the blue dots). The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a mobile 'sea' of electrons. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. The sea of electrons model. The sea of electrons surrounding the. Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. This electron sea model accounts for most physical.
From www.youtube.com
Electron Sea Model Metallic Bond Properties Of Metals And Non Iron Electron Sea Model The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons (the blue dots). The sea of electrons model. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. In contrast to electrons that participate in both ionic and covalent bonds, electrons that participate in metallic bonds. Iron Electron Sea Model.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID8799945 Iron Electron Sea Model The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. In contrast to electrons that participate in both ionic and covalent bonds, electrons that participate in metallic bonds delocalize, forming a sea of electrons around the positive. Metallic bonds are formed between two metal atoms. The sea of electrons model.. Iron Electron Sea Model.
From aawesseaa.blogspot.com
Electron Sea Model Definition Chemistry Iron Electron Sea Model The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. This electron sea model accounts for most physical. The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a mobile 'sea' of electrons. In contrast to electrons that participate in. Iron Electron Sea Model.
From aawesseaa.blogspot.com
Electron Sea Model Definition Iron Electron Sea Model Metallic bonds are formed between two metal atoms. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. The electron sea model positive atomic nuclei surrounded by a sea of. Iron Electron Sea Model.
From www.slideserve.com
PPT Metallic Bonding 7.3 PowerPoint Presentation, free download ID Iron Electron Sea Model A simplified model to describe metallic bonding has been developed by paul drüde. The sea of electrons surrounding the. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a. Iron Electron Sea Model.
From ria-majumdar.hubpages.com
Primary and Secondary Bonds Iron Electron Sea Model In contrast to electrons that participate in both ionic and covalent bonds, electrons that participate in metallic bonds delocalize, forming a sea of electrons around the positive. The sea of electrons surrounding the. The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a mobile 'sea' of electrons. Metallic bonds. Iron Electron Sea Model.
From www.shutterstock.com
Structure Metallic Bond Electron Sea Model Stock Vector (Royalty Free Iron Electron Sea Model The sea of electrons model. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. This electron sea model accounts for most physical. The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons (the blue dots). Metallic bonds are formed between two metal atoms. In contrast. Iron Electron Sea Model.
From www.youtube.com
Free Electron Theory Electron Sea Model Theories of Bonding in Iron Electron Sea Model Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. This electron sea model accounts for most physical. The sea of electrons model. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. The electron sea model positive atomic nuclei. Iron Electron Sea Model.
From www.pinterest.com
Electron Sea Model Easy Science Electron sea model, Electrons, Easy Iron Electron Sea Model Metallic bonds are formed between two metal atoms. Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. The sea of electrons surrounding the. The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons (the blue dots). In contrast to electrons that participate in both ionic. Iron Electron Sea Model.
From www.youtube.com
Metallic Bond Electron Sea Model Factors Affecting Metallic Bond Iron Electron Sea Model A simplified model to describe metallic bonding has been developed by paul drüde. In contrast to electrons that participate in both ionic and covalent bonds, electrons that participate in metallic bonds delocalize, forming a sea of electrons around the positive. Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together.. Iron Electron Sea Model.
From aawesseaa.blogspot.com
Electron Sea Model Definition Iron Electron Sea Model The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons (the blue dots). The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. In contrast to electrons that participate in both ionic and covalent bonds, electrons that participate in metallic bonds delocalize, forming a sea of. Iron Electron Sea Model.
From www.slideserve.com
PPT Metallic Bonding PowerPoint Presentation, free download ID711524 Iron Electron Sea Model Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. The sea of electrons model. The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a mobile 'sea' of electrons. In contrast to electrons that participate in both ionic and. Iron Electron Sea Model.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID5824237 Iron Electron Sea Model The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a mobile 'sea' of electrons. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. This electron sea model accounts for most physical. The sea of electrons surrounding the. The. Iron Electron Sea Model.
From devinitionva.blogspot.com
Definition Of Electron Sea Model DEFINITIONVA Iron Electron Sea Model The sea of electrons surrounding the. This electron sea model accounts for most physical. A simplified model to describe metallic bonding has been developed by paul drüde. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. Such an attractive force between the negatively charged electrons and metal cations is. Iron Electron Sea Model.
From devinitionva.blogspot.com
Definition Of Electron Sea Model DEFINITIONVA Iron Electron Sea Model In contrast to electrons that participate in both ionic and covalent bonds, electrons that participate in metallic bonds delocalize, forming a sea of electrons around the positive. The sea of electrons surrounding the. The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a mobile 'sea' of electrons. This electron. Iron Electron Sea Model.
From www.slideserve.com
PPT BONDING PowerPoint Presentation, free download ID5943045 Iron Electron Sea Model The sea of electrons model. This electron sea model accounts for most physical. A simplified model to describe metallic bonding has been developed by paul drüde. The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons (the blue dots). The sea of electrons surrounding the. Such an attractive force between the negatively charged electrons and metal. Iron Electron Sea Model.
From www.youtube.com
METALLIC BOND ELECTRON SEA MODEL YouTube Iron Electron Sea Model The sea of electrons surrounding the. In contrast to electrons that participate in both ionic and covalent bonds, electrons that participate in metallic bonds delocalize, forming a sea of electrons around the positive. Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. This electron sea model accounts for most. Iron Electron Sea Model.
From www.youtube.com
Metallic Bonding and Metallic Properties Explained Electron Sea Model Iron Electron Sea Model The sea of electrons model. In contrast to electrons that participate in both ionic and covalent bonds, electrons that participate in metallic bonds delocalize, forming a sea of electrons around the positive. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. Such an attractive force between the negatively charged. Iron Electron Sea Model.
From www.slideshare.net
Ch 8 Ionic Compounds Iron Electron Sea Model A simplified model to describe metallic bonding has been developed by paul drüde. The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a mobile 'sea' of electrons. This electron sea model accounts for most physical. The sea of electrons surrounding the. Such an attractive force between the negatively charged. Iron Electron Sea Model.
From www.youtube.com
Electrons Sea Model.mp4 YouTube Iron Electron Sea Model Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. The sea of electrons surrounding the. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. A simplified model to describe metallic bonding has been developed by paul drüde. This. Iron Electron Sea Model.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Iron Electron Sea Model A simplified model to describe metallic bonding has been developed by paul drüde. Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. This electron sea model accounts for most physical. In contrast to electrons that participate in both ionic and covalent bonds, electrons that participate in metallic bonds delocalize,. Iron Electron Sea Model.
From www.thoughtco.com
What Is the ElectronSea Model? Iron Electron Sea Model The sea of electrons model. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons (the blue dots). This electron sea model accounts for most physical. A simplified model to describe metallic bonding has been developed. Iron Electron Sea Model.
From mammothmemory.net
Metals A sea of electrons Iron Electron Sea Model The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a mobile 'sea' of electrons. A simplified model to describe metallic bonding has been developed by paul drüde. The sea of electrons surrounding the. The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons (the blue. Iron Electron Sea Model.
From www.youtube.com
What is Metallic Bond & Electron Sea Model YouTube Iron Electron Sea Model The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons (the blue dots). This electron sea model accounts for most physical. A simplified model to describe metallic bonding has been developed by paul drüde. Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. Metallic bonds. Iron Electron Sea Model.
From app.jove.com
Metallic Bond the Electron Sea Model and Properties of Metals Iron Electron Sea Model Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. A simplified model to describe metallic bonding has been developed by paul drüde. Metallic bonds are formed between two metal atoms. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout. Iron Electron Sea Model.
From www.slideserve.com
PPT 9/16/13 PowerPoint Presentation, free download ID1588141 Iron Electron Sea Model Metallic bonds are formed between two metal atoms. The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a mobile 'sea' of electrons. Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. The sea of electrons model. The electron. Iron Electron Sea Model.
From www.slideserve.com
PPT Chapter 16 PowerPoint Presentation, free download ID3913506 Iron Electron Sea Model Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. A simplified model to describe metallic bonding has been developed by paul drüde. The sea of electrons surrounding the. The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons (the blue dots). This electron sea model. Iron Electron Sea Model.
From www.slideserve.com
PPT Liquids and Solids PowerPoint Presentation, free download ID Iron Electron Sea Model The sea of electrons surrounding the. Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons (the blue dots). Metallic bonds are formed between two metal atoms. A simplified model to describe metallic bonding has been. Iron Electron Sea Model.
From questions.kunduz.com
The Electron Sea model of metalic bondin... Chemistry Iron Electron Sea Model The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a mobile 'sea' of electrons. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. The sea of electrons model. The sea of electrons surrounding the. This electron sea model. Iron Electron Sea Model.
From www.slideserve.com
PPT Covalent Bonds PowerPoint Presentation, free download ID9732055 Iron Electron Sea Model Metallic bonds are formed between two metal atoms. The sea of electrons model. The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons (the blue dots). The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a mobile 'sea' of electrons. Such an attractive force between. Iron Electron Sea Model.
From franklinkruwochoa.blogspot.com
The Electron Sea Model Is Used to Describe Bonding in FranklinkruwOchoa Iron Electron Sea Model This electron sea model accounts for most physical. A simplified model to describe metallic bonding has been developed by paul drüde. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. The sea of electrons model. The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons. Iron Electron Sea Model.
From www.slideserve.com
PPT Test 3 Review PowerPoint Presentation, free download ID2984982 Iron Electron Sea Model The electron sea model positive atomic nuclei surrounded by a sea of delocalized electrons (the blue dots). The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a mobile 'sea' of electrons. The sea of electrons model. In contrast to electrons that participate in both ionic and covalent bonds, electrons. Iron Electron Sea Model.
From shaunmwilliams.com
Chapter 10 Presentation Iron Electron Sea Model The sea of electrons model. This electron sea model accounts for most physical. Metallic bonds are formed between two metal atoms. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms. Iron Electron Sea Model.
From www.slideserve.com
PPT Chapter 23 Metals and Metallurgy PowerPoint Presentation, free Iron Electron Sea Model The sea of electrons model. This electron sea model accounts for most physical. Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. Metallic bonds are formed between two metal atoms. The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout. Iron Electron Sea Model.
From www.youtube.com
Metallic Bonding and the Electron Sea Model, Electrical Conductivity Iron Electron Sea Model The electron sea model describes metallic bonding as a system where electrons are delocalized and free to move throughout a. Such an attractive force between the negatively charged electrons and metal cations is called metallic bonds, holding the atoms together. The sea of electrons surrounding the. In contrast to electrons that participate in both ionic and covalent bonds, electrons that. Iron Electron Sea Model.