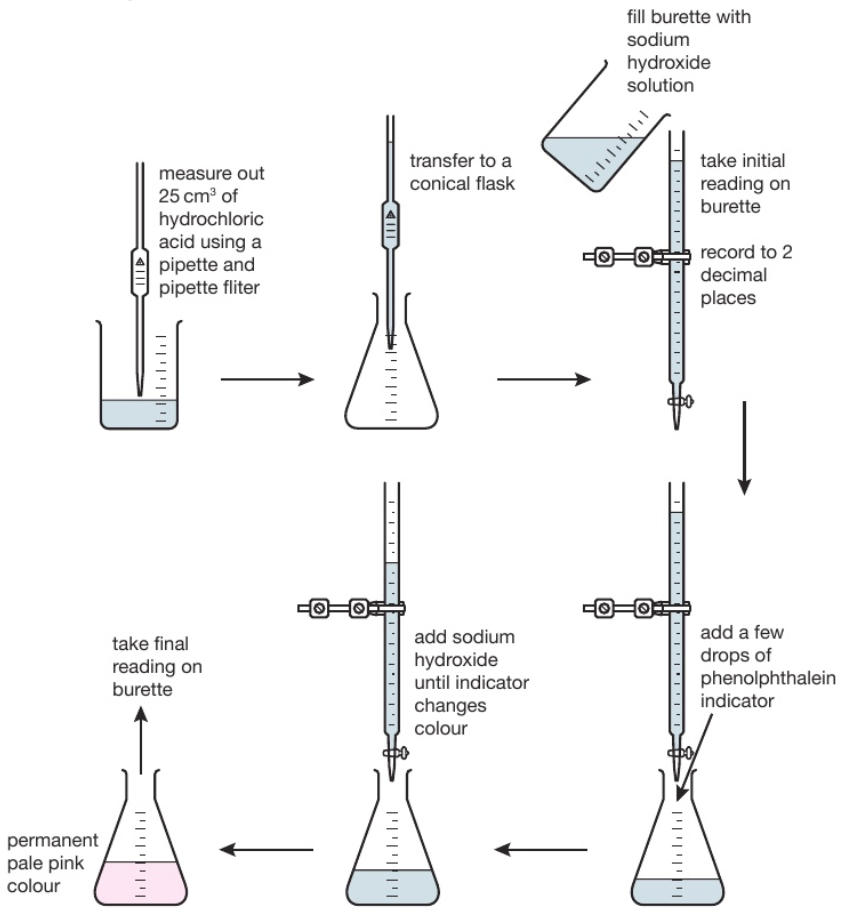
Titration Of Hcl And Na2Co3 . Titration of na₂co₃ with hcl. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. This process is known as standardising the hydrochloric acid. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl switches places with one of the sodium ions in na 2 co 3 to produce sodium hydrogencarbonate, also known as sodium bicarbonate (baking soda), and sodium chloride (salt). The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. The reaction between sodium carbonate and.
from stevenemahurino.blob.core.windows.net
Titration of na₂co₃ with hcl. This process is known as standardising the hydrochloric acid. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. The reaction between sodium carbonate and. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl switches places with one of the sodium ions in na 2 co 3 to produce sodium hydrogencarbonate, also known as sodium bicarbonate (baking soda), and sodium chloride (salt). 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid.
Acid Base Titration Na2Co3 And Hcl at stevenemahurino blog
Titration Of Hcl And Na2Co3 This process is known as standardising the hydrochloric acid. 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. Titration of na₂co₃ with hcl. This process is known as standardising the hydrochloric acid. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl switches places with one of the sodium ions in na 2 co 3 to produce sodium hydrogencarbonate, also known as sodium bicarbonate (baking soda), and sodium chloride (salt). The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. The reaction between sodium carbonate and.
From www.youtube.com
Net Ionic Equation for Na2CO3 + HCl Sodium carbonate + Hydrochloric Titration Of Hcl And Na2Co3 Today, you will use it to find the concentration of dilute hydrochloric acid by titration. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. The reaction between sodium carbonate and. Titration of na₂co₃ with hcl. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate. Titration Of Hcl And Na2Co3.
From byjus.com
in the titration of Na2CO3 by HCl using methyl orange indicator Titration Of Hcl And Na2Co3 Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. The reaction between sodium carbonate and. 25 ml. Titration Of Hcl And Na2Co3.
From www.chegg.com
Solved 1. In the process of Na2CO3 titration against HCl, Titration Of Hcl And Na2Co3 When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl switches places with one of the sodium ions in na 2 co 3 to produce sodium hydrogencarbonate, also known as sodium bicarbonate (baking soda), and sodium chloride (salt). Titration of na₂co₃ with hcl. This process is known. Titration Of Hcl And Na2Co3.
From byjus.com
18. In a mixture of NaHcO3 and Na2CO3 volume of HCl required is x ml Titration Of Hcl And Na2Co3 The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. Titration of na₂co₃ with hcl. This process is known as standardising the hydrochloric acid. 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m. Titration Of Hcl And Na2Co3.
From www.youtube.com
Na2CO3 vs HCl Titration CBSE Chemistry Practical Titration Class 11 Titration Of Hcl And Na2Co3 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl switches places with one of. Titration Of Hcl And Na2Co3.
From stevenemahurino.blob.core.windows.net
Acid Base Titration Na2Co3 And Hcl at stevenemahurino blog Titration Of Hcl And Na2Co3 The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. Titration of na₂co₃ with hcl. 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. The reaction between sodium carbonate and. When you. Titration Of Hcl And Na2Co3.
From childhealthpolicy.vumc.org
🌈 Na2co3 hcl titration. Why is used Na2CO3 with HCl in this titration Titration Of Hcl And Na2Co3 The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. This process is known as standardising the hydrochloric acid. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl switches places with one of the sodium ions. Titration Of Hcl And Na2Co3.
From www.youtube.com
Standardization Of HCl With Na2CO3; Standardization Of NaOH With Titration Of Hcl And Na2Co3 When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl switches places with one of the sodium ions in na 2 co 3 to produce sodium hydrogencarbonate, also known as sodium bicarbonate (baking soda), and sodium chloride (salt). 25 ml of 0.125 m nax2cox3 is titrated with. Titration Of Hcl And Na2Co3.
From www.chegg.com
Solved After the titration of Na2CO3 (0.1M) with HCl using Titration Of Hcl And Na2Co3 Today, you will use it to find the concentration of dilute hydrochloric acid by titration. Titration of na₂co₃ with hcl. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. Given that ka1 = 4.3 ×10−7. Titration Of Hcl And Na2Co3.
From stevenemahurino.blob.core.windows.net
Acid Base Titration Na2Co3 And Hcl at stevenemahurino blog Titration Of Hcl And Na2Co3 Titration of na₂co₃ with hcl. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. The reaction between sodium carbonate and. 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. The hydrochloric acid solution is generally titrated. Titration Of Hcl And Na2Co3.
From www.studypool.com
SOLUTION Titration na2co3 vs hcl Studypool Titration Of Hcl And Na2Co3 Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl switches places with one of the sodium. Titration Of Hcl And Na2Co3.
From www.youtube.com
Na2CO3 + HCl Sodium Carbonate + Hydrochloric Acid YouTube Titration Of Hcl And Na2Co3 Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. This process is. Titration Of Hcl And Na2Co3.
From tukioka-clinic.com
😂 Titration of naoh and na2co3 with hcl. Titration Of Hcl And Na2co3 Titration Of Hcl And Na2Co3 Today, you will use it to find the concentration of dilute hydrochloric acid by titration. 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. This process is known as standardising the hydrochloric acid. The reaction between sodium carbonate and. Titration of na₂co₃ with hcl. Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the. Titration Of Hcl And Na2Co3.
From www.chegg.com
Solved Titration Lab Report Titration of Na2CO3 with HCl Titration Of Hcl And Na2Co3 Titration of na₂co₃ with hcl. Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. The reaction between sodium carbonate and. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. The hydrochloric acid solution is generally titrated by using a methyl orange. Titration Of Hcl And Na2Co3.
From www.numerade.com
SOLVED During the double indicator titration of a mixture of Na2CO3 Titration Of Hcl And Na2Co3 Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. The reaction between sodium carbonate and. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. When you add a. Titration Of Hcl And Na2Co3.
From www.studypool.com
SOLUTION Sodium carbonate titrationhcl with hcl na2co3 Studypool Titration Of Hcl And Na2Co3 When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl switches places with one of the sodium ions in na 2 co 3 to produce sodium hydrogencarbonate, also known as sodium bicarbonate (baking soda), and sodium chloride (salt). Today, you will use it to find the concentration. Titration Of Hcl And Na2Co3.
From www.studypool.com
SOLUTION Titration na2co3 vs hcl Studypool Titration Of Hcl And Na2Co3 The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl. Titration Of Hcl And Na2Co3.
From tukioka-clinic.com
😂 Titration of naoh and na2co3 with hcl. Titration Of Hcl And Na2co3 Titration Of Hcl And Na2Co3 Titration of na₂co₃ with hcl. Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. The reaction between sodium carbonate and. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate. Titration Of Hcl And Na2Co3.
From dxohazwro.blob.core.windows.net
Titration Hcl And Na2Co3 at Madeline Arnold blog Titration Of Hcl And Na2Co3 Today, you will use it to find the concentration of dilute hydrochloric acid by titration. 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. The color of the solution changes in this titration process due to the formation of the product which. Titration Of Hcl And Na2Co3.
From www.scribd.com
Na2CO3 HCL NaOH Titration PDF Redox Titration Titration Of Hcl And Na2Co3 Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. The reaction between sodium carbonate and. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. Titration of na₂co₃ with hcl. 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. When you add a hydrochloric acid (hcl). Titration Of Hcl And Na2Co3.
From www.youtube.com
Na2CO3+HCl=NaCl+CO2+H2O Balanced EquationSodium carbonate Titration Of Hcl And Na2Co3 The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. This process is known as standardising the hydrochloric acid. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard. Titration Of Hcl And Na2Co3.
From dxohazwro.blob.core.windows.net
Titration Hcl And Na2Co3 at Madeline Arnold blog Titration Of Hcl And Na2Co3 Today, you will use it to find the concentration of dilute hydrochloric acid by titration. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. This process is known as standardising the hydrochloric acid. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2. Titration Of Hcl And Na2Co3.
From www.youtube.com
HCl vs Na2Co3 titration calculation for class11th practical Titration Of Hcl And Na2Co3 The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. The reaction between sodium carbonate and. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution.. Titration Of Hcl And Na2Co3.
From www.youtube.com
Titration of (NaOH+Na2CO3) vs HCl with Calculation of Strength, gm/lt Titration Of Hcl And Na2Co3 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. Titration of na₂co₃ with hcl. This process is known as standardising the hydrochloric acid. Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate. Titration Of Hcl And Na2Co3.
From www.youtube.com
AcidBase Titration II HCl Na2CO3 Titration II Neutralization Titration Of Hcl And Na2Co3 Today, you will use it to find the concentration of dilute hydrochloric acid by titration. The reaction between sodium carbonate and. Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. This process is known as standardising the hydrochloric acid. Titration of na₂co₃ with hcl. 25 ml of 0.125 m nax2cox3 is titrated with 0.100. Titration Of Hcl And Na2Co3.
From www.youtube.com
Lab 2 (Titration of HCl with Na2CO3) YouTube Titration Of Hcl And Na2Co3 The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. Titration of na₂co₃ with hcl. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the. Titration Of Hcl And Na2Co3.
From www.youtube.com
and Analytical Chemistry Lab. Titration of NaOH and Na2CO3 Titration Of Hcl And Na2Co3 Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. Titration of na₂co₃ with hcl. The reaction between sodium carbonate and. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. The hydrochloric acid solution is generally titrated by using a methyl orange. Titration Of Hcl And Na2Co3.
From www.studypool.com
SOLUTION Titration na2co3 vs hcl Studypool Titration Of Hcl And Na2Co3 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl switches places with one of the sodium ions in na 2 co 3 to produce sodium hydrogencarbonate, also known as sodium bicarbonate (baking soda), and. Titration Of Hcl And Na2Co3.
From www.youtube.com
titration of sodium carbonate with HCI titration of Na2CO3 Vs HCI Titration Of Hcl And Na2Co3 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. The reaction between sodium carbonate and. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. When you add a hydrochloric. Titration Of Hcl And Na2Co3.
From www.youtube.com
Titration between Na2CO3 and HCl using phenolphthalein and methyl Titration Of Hcl And Na2Co3 This process is known as standardising the hydrochloric acid. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl switches places with one of the sodium ions in na 2 co 3 to produce sodium hydrogencarbonate, also known as sodium bicarbonate (baking soda), and sodium chloride (salt).. Titration Of Hcl And Na2Co3.
From www.chegg.com
Solved 1. Consider the titration of Na2CO3 with HCl to Titration Of Hcl And Na2Co3 This process is known as standardising the hydrochloric acid. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. Titration of na₂co₃ with hcl. The reaction between sodium carbonate and. Given that ka1 = 4.3 ×10−7 and ka2. Titration Of Hcl And Na2Co3.
From stevenemahurino.blob.core.windows.net
Acid Base Titration Na2Co3 And Hcl at stevenemahurino blog Titration Of Hcl And Na2Co3 Titration of na₂co₃ with hcl. The reaction between sodium carbonate and. This process is known as standardising the hydrochloric acid. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. Today, you will. Titration Of Hcl And Na2Co3.
From www.scribd.com
1 Estimation of Na2CO3 and NaOH in A Mixture Using HCL PDF Titration Of Hcl And Na2Co3 This process is known as standardising the hydrochloric acid. Titration of na₂co₃ with hcl. Given that ka1 = 4.3 ×10−7 and ka2 = 4.8 ×10−11 for the diprotic acid. The reaction between sodium carbonate and. 25 ml of 0.125 m nax2cox3 is titrated with 0.100 m hcl. The color of the solution changes in this titration process due to the. Titration Of Hcl And Na2Co3.
From dxohazwro.blob.core.windows.net
Titration Hcl And Na2Co3 at Madeline Arnold blog Titration Of Hcl And Na2Co3 The reaction between sodium carbonate and. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl switches places with one of the sodium ions in na 2. Titration Of Hcl And Na2Co3.
From www.slideserve.com
PPT Typical Applications of Neutralization Titrations Elemental Titration Of Hcl And Na2Co3 When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in hcl switches places with one of the sodium ions in na 2 co 3 to produce sodium hydrogencarbonate, also known as sodium bicarbonate (baking soda), and sodium chloride (salt). 25 ml of 0.125 m nax2cox3 is titrated with. Titration Of Hcl And Na2Co3.