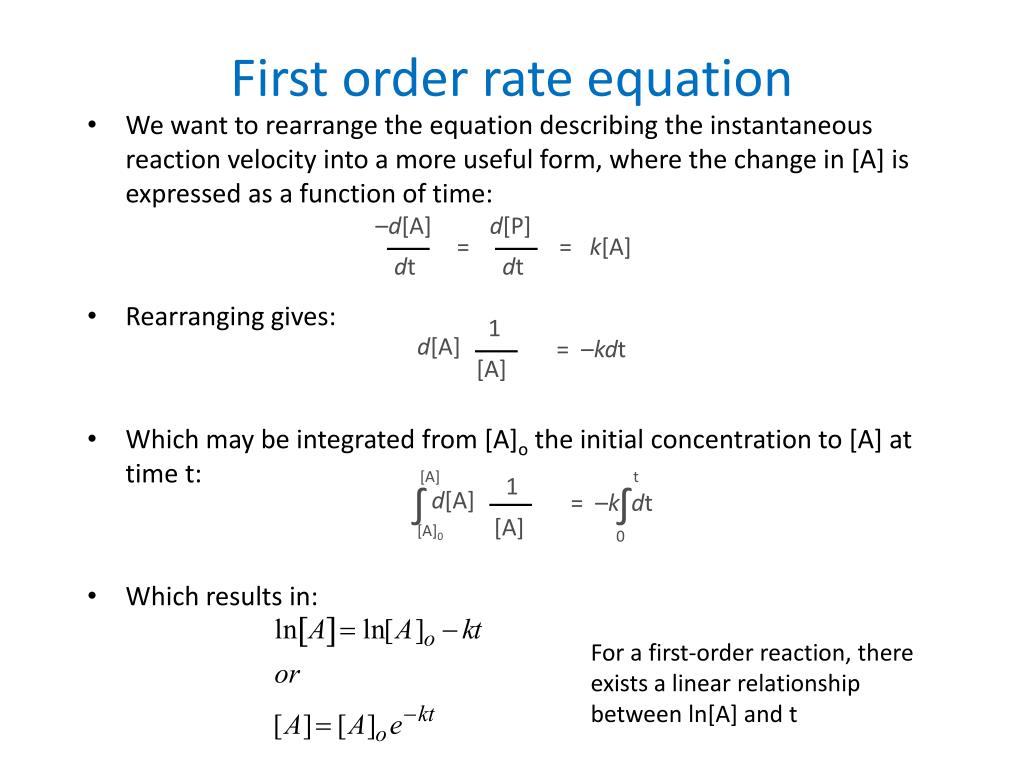
Velocity Constant For First Order Reaction . When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. The velocity equation is $$v. This reaction is of first order in [a]. The integrated rate law for a first. Consider that what is the velocity constant $k$ here? A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. What is its rate constant? In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of.
from www.slideserve.com
What is its rate constant? Consider that what is the velocity constant $k$ here? In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. This reaction is of first order in [a]. When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. The velocity equation is $$v. The integrated rate law for a first.
PPT Enzyme PowerPoint Presentation, free download ID1477171
Velocity Constant For First Order Reaction The integrated rate law for a first. This reaction is of first order in [a]. What is its rate constant? Consider that what is the velocity constant $k$ here? When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. The velocity equation is $$v. A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. The integrated rate law for a first. In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of.
From www.slideserve.com
PPT Kinematics Equations PowerPoint Presentation, free download ID Velocity Constant For First Order Reaction This reaction is of first order in [a]. Consider that what is the velocity constant $k$ here? In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. The velocity equation is $$v. When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal. Velocity Constant For First Order Reaction.
From www.tessshebaylo.com
Derive Integrated Rate Equation For First Order Reaction Tessshebaylo Velocity Constant For First Order Reaction A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. The integrated rate law for a first. The velocity equation is $$v. Consider that what is. Velocity Constant For First Order Reaction.
From www.biologyonline.com
Vmax Definition and Examples Biology Online Dictionary Velocity Constant For First Order Reaction What is its rate constant? In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. This reaction is of first order in [a]. The velocity equation is $$v. Consider that what is the velocity constant $k$ here? The integrated rate law for a first. A first. Velocity Constant For First Order Reaction.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID1477171 Velocity Constant For First Order Reaction A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. This reaction is of first order in [a]. Consider that what is the velocity constant $k$. Velocity Constant For First Order Reaction.
From www.slideserve.com
PPT Chapter 2 Enzyme PowerPoint Presentation, free download ID3561286 Velocity Constant For First Order Reaction In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. Consider that what is the velocity constant $k$ here? This reaction is of first order in [a]. The velocity equation is $$v. What is its rate constant? When \([s] >> k_m\), \[v = v_{max}\] this means. Velocity Constant For First Order Reaction.
From ar.inspiredpencil.com
First Order Reaction Graph Velocity Constant For First Order Reaction This reaction is of first order in [a]. The integrated rate law for a first. Consider that what is the velocity constant $k$ here? What is its rate constant? When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. A first order (or unimolecular) reaction then is one in which. Velocity Constant For First Order Reaction.
From www.youtube.com
AP Physics Workbook 1.F Constant Velocity YouTube Velocity Constant For First Order Reaction The integrated rate law for a first. This reaction is of first order in [a]. The velocity equation is $$v. Consider that what is the velocity constant $k$ here? In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. A first order (or unimolecular) reaction then. Velocity Constant For First Order Reaction.
From www.slideserve.com
PPT PowerPoint Presentation ID3920002 Velocity Constant For First Order Reaction In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. What is its rate constant? A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. The integrated rate law for a first.. Velocity Constant For First Order Reaction.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID7073032 Velocity Constant For First Order Reaction Consider that what is the velocity constant $k$ here? In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. This reaction is of first order in [a]. The velocity equation is $$v. A first order (or unimolecular) reaction then is one in which the velocity of. Velocity Constant For First Order Reaction.
From chem.libretexts.org
Chapter 14.4 Using Graphs to Determine Rate Laws, Rate Constants and Velocity Constant For First Order Reaction In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. Consider that what is the velocity constant $k$ here? This reaction is of first order in [a]. A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to. Velocity Constant For First Order Reaction.
From www.slideserve.com
PPT OneDimensional Motion PowerPoint Presentation, free download Velocity Constant For First Order Reaction When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. This reaction is of first order in [a]. The integrated rate law for a first. The velocity equation is $$v. In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the. Velocity Constant For First Order Reaction.
From www.slideserve.com
PPT Enzyme and Catalysis PowerPoint Presentation, free Velocity Constant For First Order Reaction Consider that what is the velocity constant $k$ here? When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. The integrated rate law for a first. In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of.. Velocity Constant For First Order Reaction.
From askfilo.com
18 If a first order reaction completed to 75 in 6 hours the velocity con.. Velocity Constant For First Order Reaction In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. The velocity equation is $$v. This reaction is of first order in [a]. Consider that what is the velocity constant $k$ here? What is its rate constant? A first order (or unimolecular) reaction then is one. Velocity Constant For First Order Reaction.
From www.youtube.com
Position and Displacement Equation (for Constant Velocity Motion Velocity Constant For First Order Reaction When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. Consider that what is the velocity constant $k$ here? What is its rate constant? This reaction is of first order in [a]. The velocity equation is $$v. The integrated rate law for a first. A first order (or unimolecular) reaction. Velocity Constant For First Order Reaction.
From askfilo.com
The unit of the velocity constant of first order reaction is Filo Velocity Constant For First Order Reaction What is its rate constant? Consider that what is the velocity constant $k$ here? This reaction is of first order in [a]. When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional. Velocity Constant For First Order Reaction.
From www.slideserve.com
PPT Reaction 1 st order reactions PowerPoint Presentation Velocity Constant For First Order Reaction In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. Consider that what is the velocity constant $k$ here? A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. The integrated rate. Velocity Constant For First Order Reaction.
From www.tessshebaylo.com
Derive Integrated Rate Equation For A First Order Gas Phase Reaction Velocity Constant For First Order Reaction The integrated rate law for a first. The velocity equation is $$v. In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant.. Velocity Constant For First Order Reaction.
From uniapaclisbon2018.com
How To Calculate Initial Velocity Of Enzyme Reaction Velocity Constant For First Order Reaction What is its rate constant? This reaction is of first order in [a]. In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. The integrated rate law for a first. A first order (or unimolecular) reaction then is one in which the velocity of the reaction. Velocity Constant For First Order Reaction.
From edurev.in
Derive an integrated rate equation for the velocity constant of a zero Velocity Constant For First Order Reaction This reaction is of first order in [a]. When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. The integrated rate law for a first. Consider. Velocity Constant For First Order Reaction.
From askfilo.com
Integrated Integrated velocity equation for first order reaction is... Velocity Constant For First Order Reaction The integrated rate law for a first. When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. The velocity equation is $$v. In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. A first order (or. Velocity Constant For First Order Reaction.
From www.youtube.com
Integrated Rate Equation for first Order Reaction First Order Velocity Constant For First Order Reaction The integrated rate law for a first. What is its rate constant? This reaction is of first order in [a]. The velocity equation is $$v. Consider that what is the velocity constant $k$ here? A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. In chemical. Velocity Constant For First Order Reaction.
From askfilo.com
If a first order reaction completed to 75 in 6 hours the velocity consta.. Velocity Constant For First Order Reaction What is its rate constant? This reaction is of first order in [a]. When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. The integrated rate law for a first. A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the. Velocity Constant For First Order Reaction.
From www.docsity.com
Constant Velocity Introduction to Physics Solved Exam Docsity Velocity Constant For First Order Reaction This reaction is of first order in [a]. What is its rate constant? A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. Consider that what is the velocity constant $k$ here? The integrated rate law for a first. When \([s] >> k_m\), \[v = v_{max}\]. Velocity Constant For First Order Reaction.
From mavink.com
First Order Reaction Units Velocity Constant For First Order Reaction This reaction is of first order in [a]. Consider that what is the velocity constant $k$ here? The integrated rate law for a first. When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. The velocity equation is $$v. A first order (or unimolecular) reaction then is one in which. Velocity Constant For First Order Reaction.
From demonstrations.wolfram.com
Reactor Rate and Conversion versus Space Velocity Wolfram Velocity Constant For First Order Reaction The velocity equation is $$v. In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. When \([s] >> k_m\), \[v = v_{max}\]. Velocity Constant For First Order Reaction.
From www.youtube.com
Biochemistry 9.2 Enzyme part 1 YouTube Velocity Constant For First Order Reaction The integrated rate law for a first. This reaction is of first order in [a]. What is its rate constant? When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the. Velocity Constant For First Order Reaction.
From studylib.net
First Order System Velocity Constant For First Order Reaction The velocity equation is $$v. A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. Consider that what is the velocity constant $k$ here? This reaction is of first order in [a]. What is its rate constant? The integrated rate law for a first. In chemical. Velocity Constant For First Order Reaction.
From the-physics-city.blogspot.com
Physics Constant Velocity Velocity Constant For First Order Reaction Consider that what is the velocity constant $k$ here? When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. The integrated rate law for a first. In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of.. Velocity Constant For First Order Reaction.
From askfilo.com
Unit of velocity constant of first order reaction is Filo Velocity Constant For First Order Reaction The integrated rate law for a first. When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. What is its rate constant? A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. Consider that what is. Velocity Constant For First Order Reaction.
From askfilo.com
Q 12. Velocity (rate) constant for a first order reaction is 7×10−45−1. C.. Velocity Constant For First Order Reaction What is its rate constant? The integrated rate law for a first. When \([s] >> k_m\), \[v = v_{max}\] this means that the rate is equal to the maximum velocity and is. Consider that what is the velocity constant $k$ here? The velocity equation is $$v. In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is. Velocity Constant For First Order Reaction.
From askfilo.com
If a first order reaction completed to 75 in 6 hours the velocity consta.. Velocity Constant For First Order Reaction Consider that what is the velocity constant $k$ here? A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. The integrated rate law for a first. In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the. Velocity Constant For First Order Reaction.
From jsiert.in
EQUATION FOR VELOCITYTIME RELATION or , First equation of Motion by Velocity Constant For First Order Reaction What is its rate constant? A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. The integrated rate law for a first. Consider that what is the velocity constant $k$ here? This reaction is of first order in [a]. In chemical kinetics, a reaction rate constant. Velocity Constant For First Order Reaction.
From www.slideserve.com
PPT Rate Laws PowerPoint Presentation, free download ID4206487 Velocity Constant For First Order Reaction What is its rate constant? In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. The integrated rate law for a first.. Velocity Constant For First Order Reaction.
From pdfsdocnts.x.fc2.com
How do identify first order reaction? Velocity Constant For First Order Reaction What is its rate constant? This reaction is of first order in [a]. Consider that what is the velocity constant $k$ here? The velocity equation is $$v. In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. A first order (or unimolecular) reaction then is one. Velocity Constant For First Order Reaction.
From researchguides.library.vanderbilt.edu
Reaction velocity over time BSCI 1510L Literature and Stats Guide Velocity Constant For First Order Reaction The integrated rate law for a first. In chemical kinetics, a reaction rate constant or reaction rate coefficient ( ) is a proportionality constant which quantifies the rate and direction of. A first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one reactant. The velocity equation is $$v.. Velocity Constant For First Order Reaction.