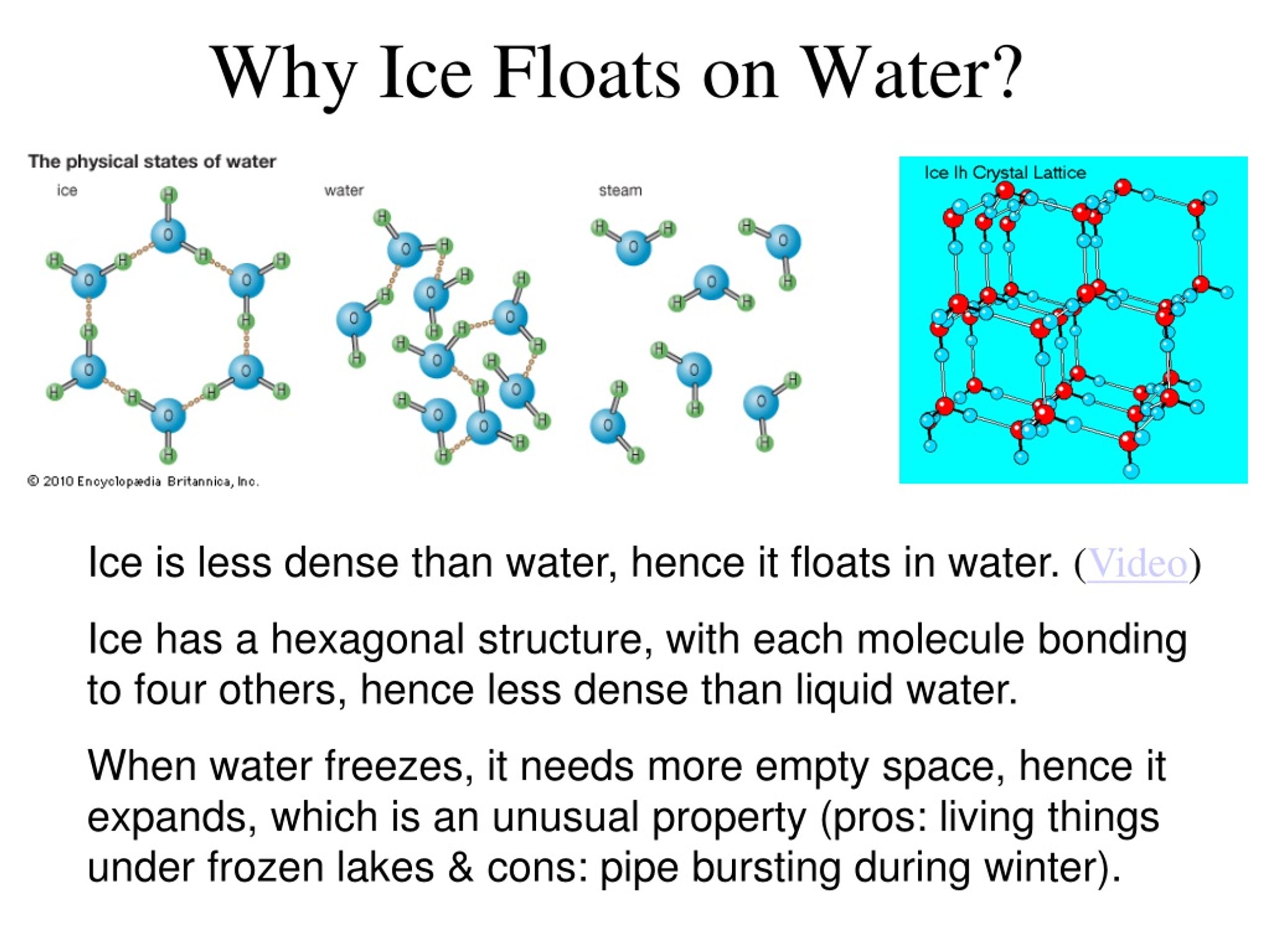
What Does Ice Float On Liquid Water . However, this is a peculiar. This is mainly because of the hydrogen bonding present in the molecules of water. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. For something to float, the upward buoyant force. So, why does ice, a solid, have a lower density than liquid water? For an object to float in water, its buoyant force has to be at least as big as its weight. The hydrogen and oxygen atoms. Why does ice float on water? The answer lies in the unique properties of water molecules and the way they behave as water transitions. As per many educational resources, the density of liquid water is around 1 g/ml. Ice floats because it is about 9% less dense than liquid water. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. The ice is lighter than the water because the density of ice is lesser than that of water. Ice cubes float because of their molecular structure.
from www.slideserve.com
Why does ice float on water? This is mainly because of the hydrogen bonding present in the molecules of water. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. Ice floats because it is about 9% less dense than liquid water. As per many educational resources, the density of liquid water is around 1 g/ml. The ice is lighter than the water because the density of ice is lesser than that of water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. So, why does ice, a solid, have a lower density than liquid water? In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water.
PPT Chapter 11 Fluid Statics PowerPoint Presentation, free download
What Does Ice Float On Liquid Water Why does ice float on water? Why does ice float on water? Ice cubes float because of their molecular structure. The ice is lighter than the water because the density of ice is lesser than that of water. The hydrogen and oxygen atoms. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. Ice floats because it is about 9% less dense than liquid water. As per many educational resources, the density of liquid water is around 1 g/ml. For an object to float in water, its buoyant force has to be at least as big as its weight. So, why does ice, a solid, have a lower density than liquid water? However, this is a peculiar. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. This is mainly because of the hydrogen bonding present in the molecules of water. For something to float, the upward buoyant force. The answer lies in the unique properties of water molecules and the way they behave as water transitions.
From www.teachoo.com
Liquids generally have lower density as compared to solids. But you What Does Ice Float On Liquid Water The ice is lighter than the water because the density of ice is lesser than that of water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. For something to float, the upward buoyant force. As per many educational resources, the density of liquid water is around 1 g/ml. It is common for us to. What Does Ice Float On Liquid Water.
From techiescientist.com
Why Does Ice Float on Water? Techiescientist What Does Ice Float On Liquid Water For an object to float in water, its buoyant force has to be at least as big as its weight. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. The answer lies in the unique properties of water molecules and the way they behave as water transitions. Why does ice float on water? Ice cubes. What Does Ice Float On Liquid Water.
From tinyhousegarage.com
Why Does Ice Float On Water Chemistry? What Does Ice Float On Liquid Water Ice floats because it is about 9% less dense than liquid water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. For an object to float in water, its buoyant force has to be at least as big as its weight. It is common for us to observe ice cubes floating when placed in a. What Does Ice Float On Liquid Water.
From exofnpcbj.blob.core.windows.net
Why Does Ice Float Hydrogen Bonds at John Thrailkill blog What Does Ice Float On Liquid Water However, this is a peculiar. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. Why does ice float on water? For. What Does Ice Float On Liquid Water.
From www.youtube.com
Does Water Float? Can you Float Liquid Water on Liquid Water? YouTube What Does Ice Float On Liquid Water The ice is lighter than the water because the density of ice is lesser than that of water. The hydrogen and oxygen atoms. Ice cubes float because of their molecular structure. Why does ice float on water? As per many educational resources, the density of liquid water is around 1 g/ml. It is common for us to observe ice cubes. What Does Ice Float On Liquid Water.
From www.youtube.com
Why ICE FLOATS on WATER YouTube What Does Ice Float On Liquid Water Ice floats because it is about 9% less dense than liquid water. The ice is lighter than the water because the density of ice is lesser than that of water. As per many educational resources, the density of liquid water is around 1 g/ml. For something to float, the upward buoyant force. For an object to float in water, its. What Does Ice Float On Liquid Water.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download What Does Ice Float On Liquid Water The hydrogen and oxygen atoms. This is mainly because of the hydrogen bonding present in the molecules of water. Ice floats because it is about 9% less dense than liquid water. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. Unlike most substances, water behaves in an. What Does Ice Float On Liquid Water.
From higheducations.com
Why Does Ice Float in Liquid Water? What Does Ice Float On Liquid Water However, this is a peculiar. For something to float, the upward buoyant force. For an object to float in water, its buoyant force has to be at least as big as its weight. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. The answer lies in the unique properties of water molecules and the way. What Does Ice Float On Liquid Water.
From slidesharenow.blogspot.com
Why Does Ice Float On Water slideshare What Does Ice Float On Liquid Water For an object to float in water, its buoyant force has to be at least as big as its weight. The hydrogen and oxygen atoms. Ice floats because it is about 9% less dense than liquid water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. The answer lies in the unique properties of water. What Does Ice Float On Liquid Water.
From www.dreamstime.com
Why Does Ice Float on Water Infographic Diagram Stock Vector What Does Ice Float On Liquid Water Why does ice float on water? It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. This is mainly because of the hydrogen bonding present in the molecules of water. However, this is a peculiar. The ice is lighter than the water because the. What Does Ice Float On Liquid Water.
From malakaianceleach.blogspot.com
Why Ice Floats on Water MalakaianceLeach What Does Ice Float On Liquid Water The ice is lighter than the water because the density of ice is lesser than that of water. However, this is a peculiar. For an object to float in water, its buoyant force has to be at least as big as its weight. For something to float, the upward buoyant force. So, why does ice, a solid, have a lower. What Does Ice Float On Liquid Water.
From www.youtube.com
Sorting Materials Into Groups Why does ice float on liquid water What Does Ice Float On Liquid Water However, this is a peculiar. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Ice cubes float because of their molecular structure. This is mainly because of the hydrogen bonding present in the molecules of water. As per many educational resources, the density of liquid water is around 1 g/ml. For an object to float. What Does Ice Float On Liquid Water.
From www.worldatlas.com
Why Does Ice Float? WorldAtlas What Does Ice Float On Liquid Water Why does ice float on water? For something to float, the upward buoyant force. Ice cubes float because of their molecular structure. Ice floats because it is about 9% less dense than liquid water. This is mainly because of the hydrogen bonding present in the molecules of water. The answer lies in the unique properties of water molecules and the. What Does Ice Float On Liquid Water.
From www.reddit.com
ELI5 Why does ice float in water? Isn't ice 'denser' than liquid water What Does Ice Float On Liquid Water Ice cubes float because of their molecular structure. The ice is lighter than the water because the density of ice is lesser than that of water. Why does ice float on water? However, this is a peculiar. The hydrogen and oxygen atoms. For something to float, the upward buoyant force. This is mainly because of the hydrogen bonding present in. What Does Ice Float On Liquid Water.
From klarayjck.blob.core.windows.net
Will Ice Always Float at Barney Carter blog What Does Ice Float On Liquid Water For something to float, the upward buoyant force. So, why does ice, a solid, have a lower density than liquid water? This is mainly because of the hydrogen bonding present in the molecules of water. Ice floats because it is about 9% less dense than liquid water. Why does ice float on water? However, this is a peculiar. It is. What Does Ice Float On Liquid Water.
From www.youtube.com
Why ice floats on liquid water? Why volume of water increases on What Does Ice Float On Liquid Water It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. Ice cubes float because of their molecular structure. Why does ice float on water? The ice is lighter than the water because the density of ice is lesser than that of water. Unlike most. What Does Ice Float On Liquid Water.
From www.branchor.com
Why does ice float on water? Exploring the science and consequences What Does Ice Float On Liquid Water However, this is a peculiar. The hydrogen and oxygen atoms. The ice is lighter than the water because the density of ice is lesser than that of water. Ice cubes float because of their molecular structure. For something to float, the upward buoyant force. It is common for us to observe ice cubes floating when placed in a glass of. What Does Ice Float On Liquid Water.
From joitwawjp.blob.core.windows.net
Why Does Ice Float On Water Solution at Jennifer Hutchison blog What Does Ice Float On Liquid Water For something to float, the upward buoyant force. So, why does ice, a solid, have a lower density than liquid water? The hydrogen and oxygen atoms. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. For an object to float in water, its buoyant force has to. What Does Ice Float On Liquid Water.
From klarayjck.blob.core.windows.net
Will Ice Always Float at Barney Carter blog What Does Ice Float On Liquid Water The answer lies in the unique properties of water molecules and the way they behave as water transitions. The hydrogen and oxygen atoms. For an object to float in water, its buoyant force has to be at least as big as its weight. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a. What Does Ice Float On Liquid Water.
From joitwawjp.blob.core.windows.net
Why Does Ice Float On Water Solution at Jennifer Hutchison blog What Does Ice Float On Liquid Water The ice is lighter than the water because the density of ice is lesser than that of water. Why does ice float on water? However, this is a peculiar. This is mainly because of the hydrogen bonding present in the molecules of water. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a. What Does Ice Float On Liquid Water.
From www.youtube.com
Why does ice float in water? Zaidan and Charles Morton YouTube What Does Ice Float On Liquid Water For something to float, the upward buoyant force. This is mainly because of the hydrogen bonding present in the molecules of water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas. What Does Ice Float On Liquid Water.
From www.youtube.com
Why does ice float on water? YouTube What Does Ice Float On Liquid Water In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. This is mainly because of the hydrogen bonding present in the molecules of water. The hydrogen and oxygen atoms. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. As per many educational resources,. What Does Ice Float On Liquid Water.
From www.scienceabc.com
Why Does Ice Float On Water? » ScienceABC What Does Ice Float On Liquid Water For an object to float in water, its buoyant force has to be at least as big as its weight. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Ice floats because it. What Does Ice Float On Liquid Water.
From techiescientist.com
Why Does Ice Float on Water? Techiescientist What Does Ice Float On Liquid Water Ice cubes float because of their molecular structure. So, why does ice, a solid, have a lower density than liquid water? As per many educational resources, the density of liquid water is around 1 g/ml. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. The hydrogen and. What Does Ice Float On Liquid Water.
From www.youtube.com
Why does ice float on water? Detailed Explanation YouTube What Does Ice Float On Liquid Water This is mainly because of the hydrogen bonding present in the molecules of water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. For an object to float in water, its buoyant force has to be at least as big as its weight. As per many educational resources, the density of liquid water is around. What Does Ice Float On Liquid Water.
From www.expii.com
Density of Water — Comparison of Solid vs. Liquid Expii What Does Ice Float On Liquid Water However, this is a peculiar. The ice is lighter than the water because the density of ice is lesser than that of water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. In. What Does Ice Float On Liquid Water.
From www.slideserve.com
PPT Ch 1 The Chemistry of Life PowerPoint Presentation, free What Does Ice Float On Liquid Water However, this is a peculiar. This is mainly because of the hydrogen bonding present in the molecules of water. It is common for us to observe ice cubes floating when placed in a glass of water, and icebergs floating on the surface seas and oceans. For an object to float in water, its buoyant force has to be at least. What Does Ice Float On Liquid Water.
From answerdbmaligner.z21.web.core.windows.net
How Does Ice Float What Does Ice Float On Liquid Water For an object to float in water, its buoyant force has to be at least as big as its weight. As per many educational resources, the density of liquid water is around 1 g/ml. Ice cubes float because of their molecular structure. In other words, ice takes up about 9% more space than water, so a liter of ice weighs. What Does Ice Float On Liquid Water.
From academichelp.net
Why Does Ice Float on Water? The Science Behind It Explained What Does Ice Float On Liquid Water For something to float, the upward buoyant force. Why does ice float on water? A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Ice cubes float because of their molecular structure. Ice floats because it is about 9% less dense than liquid water. So, why does ice, a solid, have a lower density than liquid. What Does Ice Float On Liquid Water.
From www.slideserve.com
PPT Chapter 11 Fluid Statics PowerPoint Presentation, free download What Does Ice Float On Liquid Water Ice floats because it is about 9% less dense than liquid water. The ice is lighter than the water because the density of ice is lesser than that of water. Why does ice float on water? As per many educational resources, the density of liquid water is around 1 g/ml. This is mainly because of the hydrogen bonding present in. What Does Ice Float On Liquid Water.
From www.wonderopolis.org
Why Does Ice Float in Water? Wonderopolis What Does Ice Float On Liquid Water Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. In other words, ice takes up about 9% more space than water, so a liter of ice weighs less than liter water. However, this is a peculiar. As per many educational resources, the density of liquid water is. What Does Ice Float On Liquid Water.
From thevigyan.com
Why Does Ice Float in Water? The Vigyan What Does Ice Float On Liquid Water This is mainly because of the hydrogen bonding present in the molecules of water. For an object to float in water, its buoyant force has to be at least as big as its weight. The answer lies in the unique properties of water molecules and the way they behave as water transitions. However, this is a peculiar. Why does ice. What Does Ice Float On Liquid Water.
From loeqwidny.blob.core.windows.net
Why Does Ice Floats On Water Class 9 at Lynda Evans blog What Does Ice Float On Liquid Water As per many educational resources, the density of liquid water is around 1 g/ml. This is mainly because of the hydrogen bonding present in the molecules of water. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. The hydrogen and oxygen atoms. However, this is a peculiar.. What Does Ice Float On Liquid Water.
From www.pinterest.com
Why does ice float in water? Zaidan and Charles Morton What Does Ice Float On Liquid Water Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. Ice cubes float because of their molecular structure. In other words, ice takes up about 9% more space than water, so a liter of. What Does Ice Float On Liquid Water.
From sealevel.nasa.gov
Melting Ocean Ice Affects Sea Level Unlike Ice Cubes in a Glass What Does Ice Float On Liquid Water Ice cubes float because of their molecular structure. Why does ice float on water? The hydrogen and oxygen atoms. Ice floats because it is about 9% less dense than liquid water. A water molecule (h2o) is made of two hydrogen atoms and one oxygen atom. The answer lies in the unique properties of water molecules and the way they behave. What Does Ice Float On Liquid Water.