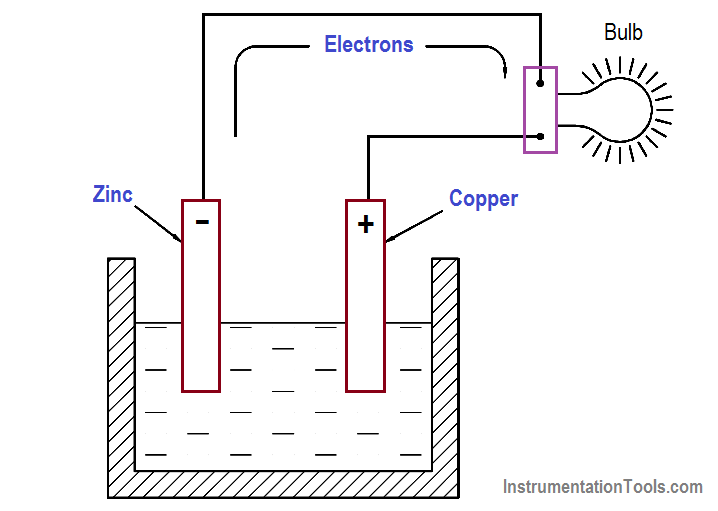
Battery Supplies Electrons . The difference in charge causes electrons to move through the wire towards the positive terminal of the battery, where they are removed from the wire. When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy. A battery is a device that stores chemical energy, and converts it to electricity. An external source of direct electrical current supplies electrons to the anode and removes them from the cathode, forcing. There are three main components of a battery: This is known as electrochemistry and the system that underpins a battery is. At the same time, the. Two terminals made of different chemicals (typically metals), the anode and the.
from instrumentationtools.com
Two terminals made of different chemicals (typically metals), the anode and the. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy. There are three main components of a battery: When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. An external source of direct electrical current supplies electrons to the anode and removes them from the cathode, forcing. The difference in charge causes electrons to move through the wire towards the positive terminal of the battery, where they are removed from the wire. A battery is a device that stores chemical energy, and converts it to electricity. At the same time, the. This is known as electrochemistry and the system that underpins a battery is.
Batteries Theory Inst Tools
Battery Supplies Electrons At the same time, the. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy. This is known as electrochemistry and the system that underpins a battery is. Two terminals made of different chemicals (typically metals), the anode and the. When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. There are three main components of a battery: An external source of direct electrical current supplies electrons to the anode and removes them from the cathode, forcing. A battery is a device that stores chemical energy, and converts it to electricity. At the same time, the. The difference in charge causes electrons to move through the wire towards the positive terminal of the battery, where they are removed from the wire.
From www.chegg.com
Solved A battery supplies electrons to the circuits. When it Battery Supplies Electrons When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. The difference in charge causes electrons to move through the wire towards the positive terminal of the battery, where they are removed from the wire. A battery is a device that stores chemical energy,. Battery Supplies Electrons.
From www.newscientist.com
Building the battery of the future today New Scientist Battery Supplies Electrons A battery is a device that stores chemical energy, and converts it to electricity. An external source of direct electrical current supplies electrons to the anode and removes them from the cathode, forcing. This is known as electrochemistry and the system that underpins a battery is. Two terminals made of different chemicals (typically metals), the anode and the. At the. Battery Supplies Electrons.
From slideplayer.com
Electric Current Chapter ppt download Battery Supplies Electrons At the same time, the. A battery is a device that stores chemical energy, and converts it to electricity. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy. When you connect a battery to a wire, the electrons in the wire are moved by. Battery Supplies Electrons.
From skill-lync.com
Week 1 Understanding Different Battery Chemistry SkillLync Battery Supplies Electrons At the same time, the. When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. There are three main components of a battery: This is known as electrochemistry and the system that underpins a battery is. Two terminals made of different chemicals (typically metals),. Battery Supplies Electrons.
From synapse-invest.ch
Hydrogen & Electrons Batteries In The Spotlight Amid The Oil Crisis Battery Supplies Electrons There are three main components of a battery: Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy. When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. An. Battery Supplies Electrons.
From www.pinterest.com
How A Battery Works Chemical reactions, It works, Learning Battery Supplies Electrons This is known as electrochemistry and the system that underpins a battery is. An external source of direct electrical current supplies electrons to the anode and removes them from the cathode, forcing. A battery is a device that stores chemical energy, and converts it to electricity. Two terminals made of different chemicals (typically metals), the anode and the. The difference. Battery Supplies Electrons.
From www.batterypowertips.com
Liion batteries, Part 4 separators Battery Power Tips Battery Supplies Electrons This is known as electrochemistry and the system that underpins a battery is. An external source of direct electrical current supplies electrons to the anode and removes them from the cathode, forcing. The difference in charge causes electrons to move through the wire towards the positive terminal of the battery, where they are removed from the wire. When you connect. Battery Supplies Electrons.
From www.researchgate.net
Schematic illustration of a lithiumion battery (LIB) under discharge Battery Supplies Electrons Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy. This is known as electrochemistry and the system that underpins a battery is. A battery is a device that stores chemical energy, and converts it to electricity. The difference in charge causes electrons to move. Battery Supplies Electrons.
From instrumentationtools.com
Batteries Theory Inst Tools Battery Supplies Electrons There are three main components of a battery: The difference in charge causes electrons to move through the wire towards the positive terminal of the battery, where they are removed from the wire. This is known as electrochemistry and the system that underpins a battery is. An external source of direct electrical current supplies electrons to the anode and removes. Battery Supplies Electrons.
From arstechnica.com
New electrode material could lead to rechargeable sodium batteries Battery Supplies Electrons Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy. Two terminals made of different chemicals (typically metals), the anode and the. At the same time, the. An external source of direct electrical current supplies electrons to the anode and removes them from the cathode,. Battery Supplies Electrons.
From www.dailymail.co.uk
Magnesium could be the key to batteries that don't explode Daily Mail Battery Supplies Electrons Two terminals made of different chemicals (typically metals), the anode and the. There are three main components of a battery: This is known as electrochemistry and the system that underpins a battery is. When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. A. Battery Supplies Electrons.
From techcentral.co.za
Explainer How lithiumion batteries work TechCentral Battery Supplies Electrons When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. Two terminals made of different chemicals (typically metals), the anode and the. The difference in charge causes electrons to move through the wire towards the positive terminal of the battery, where they are removed. Battery Supplies Electrons.
From www.pinterest.com
Figure 1 In a battery, the flow of electrons goes from anode to Battery Supplies Electrons Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy. This is known as electrochemistry and the system that underpins a battery is. A battery is a device that stores chemical energy, and converts it to electricity. When you connect a battery to a wire,. Battery Supplies Electrons.
From thepowerfacts.com
How Do Batteries Create Electricity? Here is the Reaction! The Power Battery Supplies Electrons At the same time, the. This is known as electrochemistry and the system that underpins a battery is. There are three main components of a battery: The difference in charge causes electrons to move through the wire towards the positive terminal of the battery, where they are removed from the wire. An external source of direct electrical current supplies electrons. Battery Supplies Electrons.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Battery Supplies Electrons This is known as electrochemistry and the system that underpins a battery is. There are three main components of a battery: A battery is a device that stores chemical energy, and converts it to electricity. An external source of direct electrical current supplies electrons to the anode and removes them from the cathode, forcing. Scientists are using new tools to. Battery Supplies Electrons.
From www.storyofmathematics.com
How many electrons per second enter the Positive End of battery 2? Battery Supplies Electrons This is known as electrochemistry and the system that underpins a battery is. A battery is a device that stores chemical energy, and converts it to electricity. When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. Two terminals made of different chemicals (typically. Battery Supplies Electrons.
From www.autonews.com
A look at how lithium ion batteries work Automotive News Battery Supplies Electrons This is known as electrochemistry and the system that underpins a battery is. An external source of direct electrical current supplies electrons to the anode and removes them from the cathode, forcing. At the same time, the. The difference in charge causes electrons to move through the wire towards the positive terminal of the battery, where they are removed from. Battery Supplies Electrons.
From blog.upsbatterycenter.com
The Flow of Electrons Inside Battery Cells News about Energy Storage Battery Supplies Electrons This is known as electrochemistry and the system that underpins a battery is. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy. An external source of direct electrical current supplies electrons to the anode and removes them from the cathode, forcing. When you connect. Battery Supplies Electrons.
From quizlet.com
Your friend says that a battery supplies the electrons in an Quizlet Battery Supplies Electrons An external source of direct electrical current supplies electrons to the anode and removes them from the cathode, forcing. Two terminals made of different chemicals (typically metals), the anode and the. A battery is a device that stores chemical energy, and converts it to electricity. The difference in charge causes electrons to move through the wire towards the positive terminal. Battery Supplies Electrons.
From www.automoblog.net
Toyota Scientists Discover Advanced Battery Charging Battery Supplies Electrons Two terminals made of different chemicals (typically metals), the anode and the. A battery is a device that stores chemical energy, and converts it to electricity. At the same time, the. The difference in charge causes electrons to move through the wire towards the positive terminal of the battery, where they are removed from the wire. An external source of. Battery Supplies Electrons.
From fphoto.photoshelter.com
science electricity simple circuit battery Fundamental Photographs Battery Supplies Electrons This is known as electrochemistry and the system that underpins a battery is. A battery is a device that stores chemical energy, and converts it to electricity. The difference in charge causes electrons to move through the wire towards the positive terminal of the battery, where they are removed from the wire. Scientists are using new tools to better understand. Battery Supplies Electrons.
From opentextbc.ca
17.5 Batteries and Fuel Cells Chemistry Battery Supplies Electrons This is known as electrochemistry and the system that underpins a battery is. A battery is a device that stores chemical energy, and converts it to electricity. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy. The difference in charge causes electrons to move. Battery Supplies Electrons.
From chargedevs.com
Charged EVs Researchers demonstrate a fourelectron reaction that Battery Supplies Electrons A battery is a device that stores chemical energy, and converts it to electricity. This is known as electrochemistry and the system that underpins a battery is. When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. Scientists are using new tools to better. Battery Supplies Electrons.
From cosmosmagazine.com
How do batteries work? Battery Supplies Electrons There are three main components of a battery: At the same time, the. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy. A battery is a device that stores chemical energy, and converts it to electricity. The difference in charge causes electrons to move. Battery Supplies Electrons.
From www.linuxfriends.net
Solar Energy Introduction Course Battery Supplies Electrons Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy. When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. At the same time, the. This is known as. Battery Supplies Electrons.
From socratic.org
Your friend says that a battery supplies the electrons in an electric Battery Supplies Electrons This is known as electrochemistry and the system that underpins a battery is. When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of. Battery Supplies Electrons.
From www.slideserve.com
PPT Electrons flow from the __ terminal of the battery, through the Battery Supplies Electrons An external source of direct electrical current supplies electrons to the anode and removes them from the cathode, forcing. There are three main components of a battery: The difference in charge causes electrons to move through the wire towards the positive terminal of the battery, where they are removed from the wire. When you connect a battery to a wire,. Battery Supplies Electrons.
From www.jeh-tech.com
Batteries notes Battery Supplies Electrons A battery is a device that stores chemical energy, and converts it to electricity. There are three main components of a battery: When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. An external source of direct electrical current supplies electrons to the anode. Battery Supplies Electrons.
From www.takomabattery.com
How do batteries work to generate sufficient power for devices? The Battery Supplies Electrons This is known as electrochemistry and the system that underpins a battery is. An external source of direct electrical current supplies electrons to the anode and removes them from the cathode, forcing. When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. The difference. Battery Supplies Electrons.
From stock.adobe.com
Dry cell. Cross section of battery with cathode, anode, paste, light Battery Supplies Electrons Scientists are using new tools to better understand the electrical and chemical processes in batteries to produce a new generation of highly efficient, electrical energy. Two terminals made of different chemicals (typically metals), the anode and the. There are three main components of a battery: An external source of direct electrical current supplies electrons to the anode and removes them. Battery Supplies Electrons.
From www.alamy.com
Liion battery diagram. Vector illustration. Rechargeable battery in Battery Supplies Electrons This is known as electrochemistry and the system that underpins a battery is. An external source of direct electrical current supplies electrons to the anode and removes them from the cathode, forcing. There are three main components of a battery: A battery is a device that stores chemical energy, and converts it to electricity. Scientists are using new tools to. Battery Supplies Electrons.
From www.pinterest.com
The chemical reactions in the battery causes a build up of electrons at Battery Supplies Electrons Two terminals made of different chemicals (typically metals), the anode and the. An external source of direct electrical current supplies electrons to the anode and removes them from the cathode, forcing. There are three main components of a battery: The difference in charge causes electrons to move through the wire towards the positive terminal of the battery, where they are. Battery Supplies Electrons.
From www.electricity-magnetism.org
VLA Battery Characteristics, Applications, Pros & Cons Battery Supplies Electrons A battery is a device that stores chemical energy, and converts it to electricity. An external source of direct electrical current supplies electrons to the anode and removes them from the cathode, forcing. When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. Two. Battery Supplies Electrons.
From www.electricity-magnetism.org
Lithiumion Battery How it works Reaction, Anode & Cathode Battery Supplies Electrons When you connect a battery to a wire, the electrons in the wire are moved by the electric field provided by the emf of the battery. The difference in charge causes electrons to move through the wire towards the positive terminal of the battery, where they are removed from the wire. Two terminals made of different chemicals (typically metals), the. Battery Supplies Electrons.