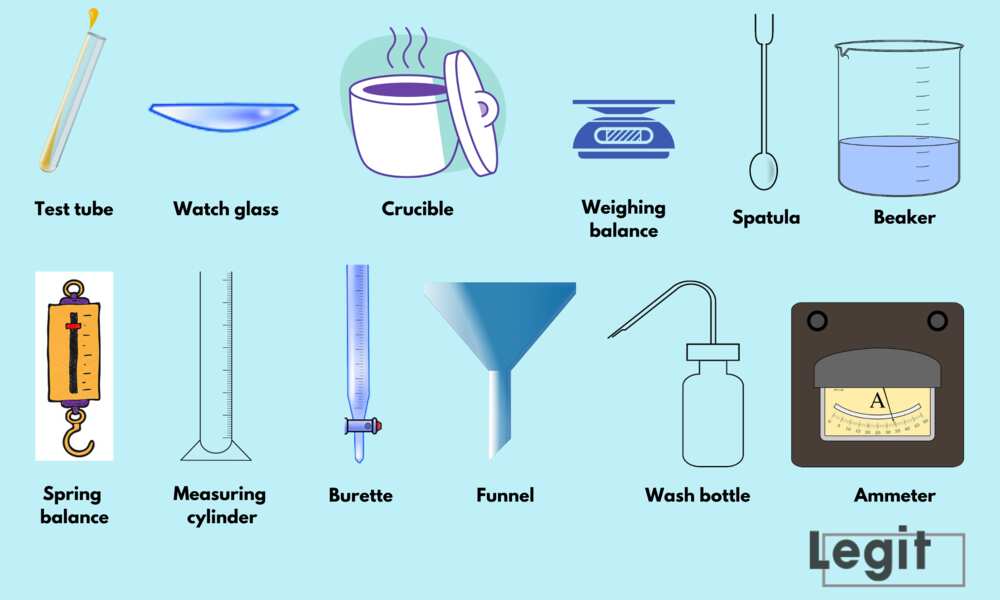
Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization . In this experiment, you will measure the heat of neutralization when an acid and base react. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. The apparatus is the calorimeter. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. We then used this calculation to find the. The heat released or consumed by the reactions will allow for the. The symbol δh is used to denote the enthalpy change. Calorimetry is the process by which the heat in a chemical or physical process can be measured. The experimental apparatus consists of a. Calorimetry is a scientific term dealing with the changes in energy of the system by measuring the heat exchanged with the surroundings.
from www.legit.ng
The heat released or consumed by the reactions will allow for the. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction. The apparatus is the calorimeter. We then used this calculation to find the. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. The symbol δh is used to denote the enthalpy change. Calorimetry is a scientific term dealing with the changes in energy of the system by measuring the heat exchanged with the surroundings. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. Calorimetry is the process by which the heat in a chemical or physical process can be measured. The experimental apparatus consists of a.
20 most common lab equipment names and their uses plus pictures
Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization The apparatus is the calorimeter. Calorimetry is a scientific term dealing with the changes in energy of the system by measuring the heat exchanged with the surroundings. Calorimetry is the process by which the heat in a chemical or physical process can be measured. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. The experimental apparatus consists of a. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. The heat released or consumed by the reactions will allow for the. The symbol δh is used to denote the enthalpy change. In this experiment, you will measure the heat of neutralization when an acid and base react. The apparatus is the calorimeter. We then used this calculation to find the. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction.
From www.pinterest.co.uk
Lab equipment and uses Common lab equipment names Laboratory Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization We then used this calculation to find the. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. Calorimetry is a scientific term dealing with the. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From grammarvocab.com
List of Lab Equipment for Chemistry Names, Uses and Pictures GrammarVocab Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. Calorimetry is the process by which the heat in a chemical or physical process can be measured. The heat released or consumed by the reactions will allow for the. The experimental apparatus consists of a.. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From klaygikyr.blob.core.windows.net
Laboratory Apparatus Names And Functions at Jeff Cadorette blog Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization The heat released or consumed by the reactions will allow for the. The symbol δh is used to denote the enthalpy change. In this experiment, you will measure the heat of neutralization when an acid and base react. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From byjus.com
Draw a labelled diagram of the apparatus you would use to determine the Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization We then used this calculation to find the. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. The apparatus is the calorimeter. Calorimetry is the process by which the heat in a chemical or physical process can be measured. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From www.transformationtutoring.com
Given the diagram of a laboratory apparatus Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization In this experiment, you will measure the heat of neutralization when an acid and base react. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction. The heat released or consumed by the reactions will allow for the. The apparatus is. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From chemistrypals.blogspot.com
Chemistry Pals COMMON LABORATORY APPARATUS Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization We then used this calculation to find the. Calorimetry is a scientific term dealing with the changes in energy of the system by measuring the heat exchanged with the surroundings. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction. The. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From depjstloeco.blob.core.windows.net
Laboratory Apparatus And Their Uses In Chemistry at Robin Fidler blog Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization Calorimetry is a scientific term dealing with the changes in energy of the system by measuring the heat exchanged with the surroundings. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From www.chegg.com
REPORT SHEET EXPERIMENT 28 Heat of Neutralization °C Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization The heat released or consumed by the reactions will allow for the. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction. The apparatus is the calorimeter. We used a constructed calorimeter and thermometer to measure the temperature of deionized water. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From infinigeek.com
Chemistry Convos 5 Common Laboratory Apparatus Names (And Their Uses Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. Calorimetry is the process by which the heat in a chemical or physical process can be measured. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From dxoifsakh.blob.core.windows.net
Label The Apparatus Used In Chemistry Laboratory at Leigh Charles blog Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. In this experiment, you will measure the heat of neutralization when an acid and base react.. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From grammarvocab.com
List of Lab Equipment Names and Pictures PDF GrammarVocab Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction. We then used this calculation to find the. In this experiment, you will measure the heat of neutralization when an acid and base react. We used a constructed calorimeter and thermometer. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From chem2u.blogspot.com
chem2U Heat of Neutralisation Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction. The symbol δh is used to denote the enthalpy change. The experimental apparatus consists of a.. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From cbselibrary.com
how to calculate heat of neutralization of hcl and naoh Archives CBSE Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. The experimental apparatus consists of a. The apparatus is the calorimeter. In this experiment, you will measure the heat of neutralization when an acid and base react. The symbol δh is used to denote the enthalpy change. Calorimetry is the process by which the heat. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From studylib.net
Heat of Neutralization Lab Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization Calorimetry is a scientific term dealing with the changes in energy of the system by measuring the heat exchanged with the surroundings. The heat released or consumed by the reactions will allow for the. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. The apparatus is the calorimeter. The symbol δh is used to. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From cbselibrary.com
how to calculate heat of neutralization of hcl and naoh Archives CBSE Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. In this experiment, you will measure the heat of neutralization when an acid and base react. The symbol δh is used to denote the enthalpy change. The heat released or consumed by the reactions will allow for the. Calorimetry is the process by which the. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From 7esl.com
Laboratory Equipment Names in English • 7ESL Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. The heat released or consumed by the reactions will allow for the. We then used this calculation to find the. The apparatus is the calorimeter. The goal of this experiment is to determine the molar. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From klaygikyr.blob.core.windows.net
Laboratory Apparatus Names And Functions at Jeff Cadorette blog Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization We then used this calculation to find the. The apparatus is the calorimeter. The symbol δh is used to denote the enthalpy change. Calorimetry is a scientific term dealing with the changes in energy of the system by measuring the heat exchanged with the surroundings. Calorimetry is the process by which the heat in a chemical or physical process can. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From scienceisfun.netlify.app
Laboratory apparatus and their uses with pictures scienceisfun Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. Calorimetry is the process by which the heat in a chemical or physical process can be measured. Calorimetry is a scientific term dealing with the changes in energy of the system by measuring the heat. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From studylib.net
Determining the Enthalpy of a Neutralization Reaction Work Sheet Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization The heat released or consumed by the reactions will allow for the. In this experiment, you will measure the heat of neutralization when an acid and base react. Calorimetry is the process by which the heat in a chemical or physical process can be measured. The apparatus is the calorimeter. We used a constructed calorimeter and thermometer to measure the. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From www.studocu.com
Lab 6 report lab Thermochemistry Heat of Neutralization and Hess’s Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization Calorimetry is a scientific term dealing with the changes in energy of the system by measuring the heat exchanged with the surroundings. The experimental apparatus consists of a. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. Calorimetry is the process by which the heat in a chemical or physical process can be measured.. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From exoabfmik.blob.core.windows.net
Draw 50 Laboratory Apparatus And Their Uses at Manuel Dupree blog Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization We then used this calculation to find the. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. The heat released or consumed by the reactions will allow for the. The apparatus is the calorimeter. In this experiment, you will measure the heat of neutralization. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From klaygikyr.blob.core.windows.net
Laboratory Apparatus Names And Functions at Jeff Cadorette blog Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization In this experiment, you will measure the heat of neutralization when an acid and base react. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction. Calorimetry is the process by which the heat in a chemical or physical process can. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From www.vernier.com
The Enthalpy of Neutralization of Phosphoric Acid > Experiment 26 from Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization We then used this calculation to find the. The symbol δh is used to denote the enthalpy change. The apparatus is the calorimeter. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. Calorimetry is a scientific term dealing with the changes in energy of the system by measuring the heat exchanged with the surroundings.. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From www.legit.ng
20 most common lab equipment names and their uses plus pictures Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization The heat released or consumed by the reactions will allow for the. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. The symbol δh is used to denote the enthalpy change. The experimental apparatus consists of a. The apparatus is the calorimeter. Introduction to the technique of calorimetry, in which the heat evolved (given. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From doodsdpogi-science.blogspot.com
The World of Science Apparatus used for heating in the Laboratory Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization In this experiment, you will measure the heat of neutralization when an acid and base react. The heat released or consumed by the reactions will allow for the. Calorimetry is a scientific term dealing with the changes in energy of the system by measuring the heat exchanged with the surroundings. The symbol δh is used to denote the enthalpy change.. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From tomdunnacademy.org
Heat of Neutralization Lab Answer Key Revealed Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction. The apparatus is the calorimeter. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. We then used this calculation to find the. The heat released. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From klaygikyr.blob.core.windows.net
Laboratory Apparatus Names And Functions at Jeff Cadorette blog Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization We then used this calculation to find the. The heat released or consumed by the reactions will allow for the. In this experiment, you will measure the heat of neutralization when an acid and base react. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From www.numerade.com
SOLVED Text Report Form; Experiment 9 Enthalpy of Neutralization Name Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. The symbol δh is used to denote the enthalpy change. The apparatus is the calorimeter. The experimental apparatus consists of a. We then used this calculation to find the. In this experiment, you will measure. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From www.chegg.com
Solved EXPERIMENT REPORT SHEET Heat of Neutralization A. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization Calorimetry is a scientific term dealing with the changes in energy of the system by measuring the heat exchanged with the surroundings. We then used this calculation to find the. Calorimetry is the process by which the heat in a chemical or physical process can be measured. The apparatus is the calorimeter. The experimental apparatus consists of a. The heat. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From joitpkpfd.blob.core.windows.net
Lab Equipment Names And Pictures And Uses Pdf at Joan McCormick blog Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization The experimental apparatus consists of a. Calorimetry is a scientific term dealing with the changes in energy of the system by measuring the heat exchanged with the surroundings. Calorimetry is the process by which the heat in a chemical or physical process can be measured. The symbol δh is used to denote the enthalpy change. We then used this calculation. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From studylib.net
Chemistry Lab Equipment Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization The heat released or consumed by the reactions will allow for the. In this experiment, you will measure the heat of neutralization when an acid and base react. Calorimetry is the process by which the heat in a chemical or physical process can be measured. The symbol δh is used to denote the enthalpy change. The apparatus is the calorimeter.. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From exyaeuonh.blob.core.windows.net
Chemistry Equipment Names And Uses at Diane Shultz blog Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization Calorimetry is a scientific term dealing with the changes in energy of the system by measuring the heat exchanged with the surroundings. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. We then used this calculation to find the. Introduction to the technique of. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From blogofsciencestore.blogspot.com
Blog Of Science Store Common Laboratory Apparatus Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an insulated reaction. Calorimetry is the process by which the heat in a chemical or physical process can be measured. We then used this calculation to find the. The apparatus is the calorimeter. The. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From www.youtube.com
Laboratory Equipment Names and Vocabulary Instrument List In English Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization In this experiment, you will measure the heat of neutralization when an acid and base react. Calorimetry is the process by which the heat in a chemical or physical process can be measured. The heat released or consumed by the reactions will allow for the. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.
From www.numerade.com
SOLVED In a laboratory experiment to determine the enthalpy of Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization Calorimetry is a scientific term dealing with the changes in energy of the system by measuring the heat exchanged with the surroundings. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. The apparatus is the calorimeter. In this experiment, you will measure the heat of neutralization when an acid and base react. We then. Name The Apparatus Used In The Laboratory To Determine The Heat Of Neutralization.