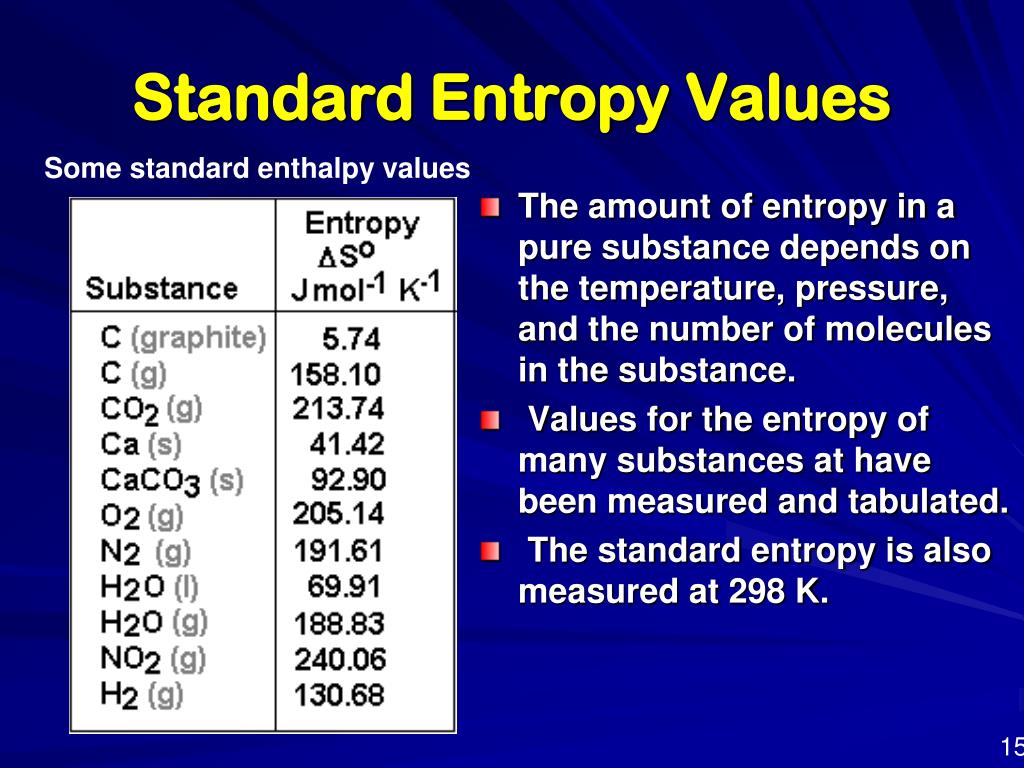
Standard Entropy Of Lithium . Most values are those given in the nbs technical notes (reference 1) after conversion. The standard state values used in this text are p=1 bar (0.983 atm), t=298k and the concentration of a solute. The standard molar entropy is the entropy contained in one mole of a substance at standard state. Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. Basing on the the standard. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds This table gives a few thermodynamic data for lithium. Pressure 1013.25 hpa and temperature 25 °c. All standard state, 25 °c and 1 bar (written to 1 decimal place). Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol) (nh 4) 2o (l).
from www.slideserve.com
Pressure 1013.25 hpa and temperature 25 °c. The standard molar entropy is the entropy contained in one mole of a substance at standard state. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds All standard state, 25 °c and 1 bar (written to 1 decimal place). This table gives a few thermodynamic data for lithium. Most values are those given in the nbs technical notes (reference 1) after conversion. The standard state values used in this text are p=1 bar (0.983 atm), t=298k and the concentration of a solute. Basing on the the standard. Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol) (nh 4) 2o (l).
PPT Entropy PowerPoint Presentation, free download ID2017581
Standard Entropy Of Lithium This table gives a few thermodynamic data for lithium. Basing on the the standard. The standard molar entropy is the entropy contained in one mole of a substance at standard state. Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol) (nh 4) 2o (l). Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. Most values are those given in the nbs technical notes (reference 1) after conversion. This table gives a few thermodynamic data for lithium. All standard state, 25 °c and 1 bar (written to 1 decimal place). Pressure 1013.25 hpa and temperature 25 °c. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds The standard state values used in this text are p=1 bar (0.983 atm), t=298k and the concentration of a solute.
From www.chegg.com
Solved Calculate the standard entropy change for the Standard Entropy Of Lithium The standard state values used in this text are p=1 bar (0.983 atm), t=298k and the concentration of a solute. Most values are those given in the nbs technical notes (reference 1) after conversion. Pressure 1013.25 hpa and temperature 25 °c. This table gives a few thermodynamic data for lithium. Basing on the the standard. All standard state, 25 °c. Standard Entropy Of Lithium.
From www.youtube.com
How to Calculate Standard Entropy of Reaction using Standard Entropy Standard Entropy Of Lithium 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds This table gives a few thermodynamic data for lithium. The standard state values used in this text are p=1 bar (0.983 atm), t=298k and the concentration of a solute. Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free. Standard Entropy Of Lithium.
From www.chegg.com
Solved Consider the following table of standard entropy Standard Entropy Of Lithium 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds The standard molar entropy is the entropy contained in one mole of a substance at standard state. Pressure 1013.25 hpa and temperature 25 °c. Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol) (nh 4) 2o. Standard Entropy Of Lithium.
From mungfali.com
Standard Entropy Chart Standard Entropy Of Lithium Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. The standard state values used in this text are p=1 bar (0.983 atm), t=298k and the concentration of a solute. Most values are those given in the nbs technical notes (reference 1) after conversion. Basing on the the. Standard Entropy Of Lithium.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Entropy Of Lithium The standard molar entropy is the entropy contained in one mole of a substance at standard state. Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. This table gives a few thermodynamic data for lithium. All standard state, 25 °c and 1 bar (written to 1 decimal. Standard Entropy Of Lithium.
From cpb.iphy.ac.cn
Entropy and heat generation of lithium cells/batteries Standard Entropy Of Lithium This table gives a few thermodynamic data for lithium. Most values are those given in the nbs technical notes (reference 1) after conversion. Basing on the the standard. Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. 100 rows standard heats and free energies of formation and. Standard Entropy Of Lithium.
From www.researchgate.net
(PDF) Review HighEntropy Materials for LithiumIon Battery Electrodes Standard Entropy Of Lithium All standard state, 25 °c and 1 bar (written to 1 decimal place). This table gives a few thermodynamic data for lithium. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds Most values are those given in the nbs technical notes (reference 1) after conversion. Standard thermodynamic values formula state of matter. Standard Entropy Of Lithium.
From www.slideserve.com
PPT Entropy and Free Energy PowerPoint Presentation, free download Standard Entropy Of Lithium Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. Basing on the the standard. Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol) (nh 4) 2o (l). The standard state values used in this text are p=1 bar (0.983 atm),. Standard Entropy Of Lithium.
From www.slideserve.com
PPT Chapter 16 Thermodynamics Entropy, Free Energy, and Equilibrium Standard Entropy Of Lithium Most values are those given in the nbs technical notes (reference 1) after conversion. This table gives a few thermodynamic data for lithium. Pressure 1013.25 hpa and temperature 25 °c. Basing on the the standard. Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. 100 rows standard. Standard Entropy Of Lithium.
From www.researchgate.net
(PDF) High entropy liquid electrolytes for lithium batteries Standard Entropy Of Lithium The standard molar entropy is the entropy contained in one mole of a substance at standard state. This table gives a few thermodynamic data for lithium. Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol) (nh 4) 2o (l). 100 rows standard heats and free energies of formation and absolute entropies of elements. Standard Entropy Of Lithium.
From mungfali.com
Enthalpies Of Formation Table Standard Entropy Of Lithium Most values are those given in the nbs technical notes (reference 1) after conversion. Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. The standard molar entropy is the entropy contained in one mole of a substance at standard state. 100 rows standard heats and free energies. Standard Entropy Of Lithium.
From www.chemistryworld.com
Entropy measurements can gauge health of lithiumion batteries Standard Entropy Of Lithium The standard molar entropy is the entropy contained in one mole of a substance at standard state. This table gives a few thermodynamic data for lithium. The standard state values used in this text are p=1 bar (0.983 atm), t=298k and the concentration of a solute. Because the entropy of a substance depends on the amount of substance, the pressure,. Standard Entropy Of Lithium.
From www.researchgate.net
Entropy values for different compounds at 298.15 K. Download Table Standard Entropy Of Lithium All standard state, 25 °c and 1 bar (written to 1 decimal place). Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. Pressure 1013.25 hpa and temperature 25 °c. The standard molar entropy is the entropy contained in one mole of a substance at standard state. This. Standard Entropy Of Lithium.
From www.researchgate.net
(a) The calculated phonon free energies of lithium silicates in which Standard Entropy Of Lithium This table gives a few thermodynamic data for lithium. Pressure 1013.25 hpa and temperature 25 °c. Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol) (nh 4) 2o (l). Most values are those given in the nbs technical notes (reference 1) after conversion. The standard molar entropy is the entropy contained in one. Standard Entropy Of Lithium.
From www.slideserve.com
PPT Entropy PowerPoint Presentation, free download ID2017581 Standard Entropy Of Lithium This table gives a few thermodynamic data for lithium. Most values are those given in the nbs technical notes (reference 1) after conversion. Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol) (nh 4) 2o (l). Basing on the the standard. Because the entropy of a substance depends on the amount of substance,. Standard Entropy Of Lithium.
From www.chinesechemsoc.org
Decimal SolventBased HighEntropy Electrolyte Enabling the Extended Standard Entropy Of Lithium Basing on the the standard. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds All standard state, 25 °c and 1 bar (written to 1 decimal place). Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. Standard thermodynamic values. Standard Entropy Of Lithium.
From www.chegg.com
Solved Using the standard entropy values in the table below, Standard Entropy Of Lithium This table gives a few thermodynamic data for lithium. All standard state, 25 °c and 1 bar (written to 1 decimal place). 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds Basing on the the standard. The standard state values used in this text are p=1 bar (0.983 atm), t=298k and the. Standard Entropy Of Lithium.
From www.chinesechemsoc.org
Decimal SolventBased HighEntropy Electrolyte Enabling the Extended Standard Entropy Of Lithium Basing on the the standard. Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol) (nh 4) 2o (l). This table gives a few thermodynamic data for lithium. Pressure 1013.25 hpa and temperature 25 °c. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds All standard. Standard Entropy Of Lithium.
From www.youtube.com
15.3.3 Calculate the standard entropy change for a reaction (HL) YouTube Standard Entropy Of Lithium Most values are those given in the nbs technical notes (reference 1) after conversion. Pressure 1013.25 hpa and temperature 25 °c. Basing on the the standard. The standard molar entropy is the entropy contained in one mole of a substance at standard state. All standard state, 25 °c and 1 bar (written to 1 decimal place). Because the entropy of. Standard Entropy Of Lithium.
From www.slideserve.com
PPT Chapter 19 Chemical Thermodynamics PowerPoint Presentation, free Standard Entropy Of Lithium Most values are those given in the nbs technical notes (reference 1) after conversion. This table gives a few thermodynamic data for lithium. The standard molar entropy is the entropy contained in one mole of a substance at standard state. Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol) (nh 4) 2o (l).. Standard Entropy Of Lithium.
From www.chegg.com
Solved Calculate ASF° for NH3(g). ASFO = JK1 Use data in Standard Entropy Of Lithium Pressure 1013.25 hpa and temperature 25 °c. Most values are those given in the nbs technical notes (reference 1) after conversion. The standard molar entropy is the entropy contained in one mole of a substance at standard state. Basing on the the standard. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds. Standard Entropy Of Lithium.
From www.mdpi.com
Batteries Free FullText On the Relations between LithiumIon Standard Entropy Of Lithium Pressure 1013.25 hpa and temperature 25 °c. Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds This table gives a few thermodynamic data for lithium. The standard state values used. Standard Entropy Of Lithium.
From wsmzaer.weebly.com
Standard entropy wsmzaer Standard Entropy Of Lithium 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds Basing on the the standard. Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol) (nh 4) 2o (l). Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it. Standard Entropy Of Lithium.
From www.w3schools.blog
Standard enthalpy of ionization W3schools Standard Entropy Of Lithium Basing on the the standard. All standard state, 25 °c and 1 bar (written to 1 decimal place). Most values are those given in the nbs technical notes (reference 1) after conversion. Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol) (nh 4) 2o (l). This table gives a few thermodynamic data for. Standard Entropy Of Lithium.
From www.researchgate.net
Molar enthalpies and entropies of intercalated lithium within the three Standard Entropy Of Lithium All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard molar entropy is the entropy contained in one mole of a substance at standard state. Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol) (nh 4) 2o (l). Most values are those given in the nbs technical notes. Standard Entropy Of Lithium.
From cpb.iphy.ac.cn
Entropy and heat generation of lithium cells/batteries Standard Entropy Of Lithium All standard state, 25 °c and 1 bar (written to 1 decimal place). This table gives a few thermodynamic data for lithium. Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol). Standard Entropy Of Lithium.
From mungfali.com
Enthalpies Of Formation Table Standard Entropy Of Lithium Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. Basing on the the standard. Pressure 1013.25 hpa and temperature 25 °c. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds This table gives a few thermodynamic data for lithium.. Standard Entropy Of Lithium.
From www.mdpi.com
Batteries Free FullText On the Relations between LithiumIon Standard Entropy Of Lithium All standard state, 25 °c and 1 bar (written to 1 decimal place). Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. Pressure 1013.25 hpa and temperature 25 °c. The standard molar entropy is the entropy contained in one mole of a substance at standard state. Most. Standard Entropy Of Lithium.
From www.researchgate.net
The standard entropy and enthalpy of reactions (lnK = ΔG°/RT, ΔG° = ΔH Standard Entropy Of Lithium Pressure 1013.25 hpa and temperature 25 °c. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard state values used in this text are p=1 bar (0.983 atm), t=298k and the concentration of a solute. Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient. Standard Entropy Of Lithium.
From www.mdpi.com
Batteries Free FullText On the Relations between LithiumIon Standard Entropy Of Lithium Pressure 1013.25 hpa and temperature 25 °c. This table gives a few thermodynamic data for lithium. The standard state values used in this text are p=1 bar (0.983 atm), t=298k and the concentration of a solute. Basing on the the standard. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds Because the. Standard Entropy Of Lithium.
From www.mdpi.com
Batteries Free FullText On the Relations between LithiumIon Standard Entropy Of Lithium The standard state values used in this text are p=1 bar (0.983 atm), t=298k and the concentration of a solute. Most values are those given in the nbs technical notes (reference 1) after conversion. Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. All standard state, 25. Standard Entropy Of Lithium.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM Chapter 16 PowerPoint Presentation, free Standard Entropy Of Lithium This table gives a few thermodynamic data for lithium. The standard molar entropy is the entropy contained in one mole of a substance at standard state. 100 rows standard heats and free energies of formation and absolute entropies of elements and inorganic compounds Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature,. Standard Entropy Of Lithium.
From onlinelibrary.wiley.com
High‐Entropy Lithium Argyrodite Solid Electrolytes Enabling Stable All Standard Entropy Of Lithium Basing on the the standard. Pressure 1013.25 hpa and temperature 25 °c. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard molar entropy is the entropy contained in one mole of a substance at standard state. This table gives a few thermodynamic data for lithium. Because the entropy of a substance depends on the. Standard Entropy Of Lithium.
From www.mdpi.com
Batteries Free FullText On the Relations between LithiumIon Standard Entropy Of Lithium All standard state, 25 °c and 1 bar (written to 1 decimal place). Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol) (nh 4) 2o (l). Pressure 1013.25 hpa and temperature 25 °c. The standard state values used in this text are p=1 bar (0.983 atm), t=298k and the concentration of a solute.. Standard Entropy Of Lithium.
From www.researchgate.net
Processed Gibbs energy and entropy parameters for Liion battery 2 Standard Entropy Of Lithium Because the entropy of a substance depends on the amount of substance, the pressure, and the temperature, it is convenient to describe. All standard state, 25 °c and 1 bar (written to 1 decimal place). Standard thermodynamic values formula state of matter enthalpy (kj/mol) entropy (j mol/k) gibbs free energy (kj/mol) (nh 4) 2o (l). The standard molar entropy is. Standard Entropy Of Lithium.