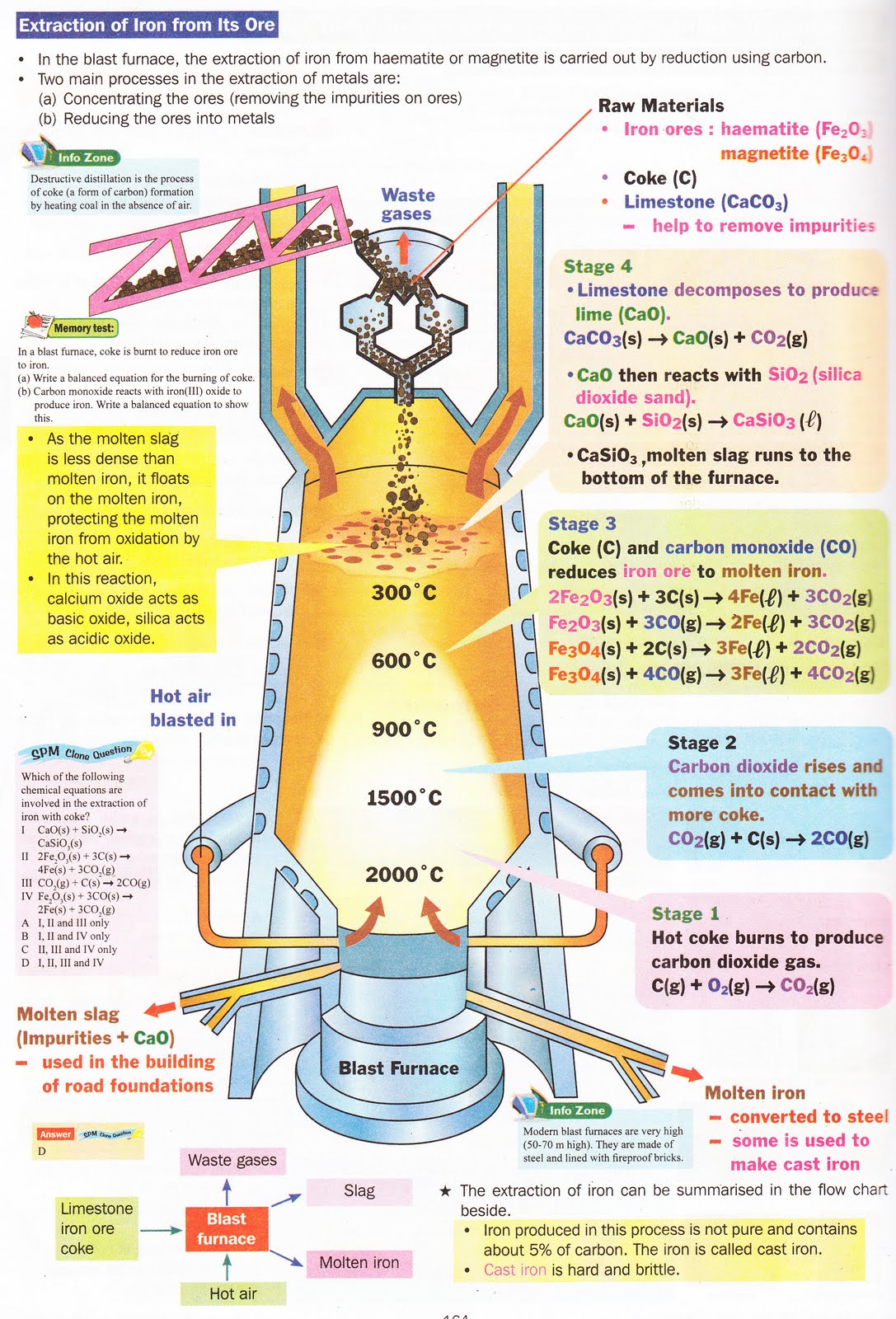
Describe How Iron Is Extracted From Its Ore . Modern blast furnaces produce approximately 10,000 tonnes of iron per day. Fran scott explains iron extraction. Iron is extracted from its ore (haematite, fe₂o₃) in a blast furnace. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the blast furnace. Iron is extracted from its ore using carbon in the form of coke. How is iron extracted from its ore? It’s a long process which begins with concentration through calcination roasting. Iron(iii) oxide is reduced to molten iron when it reacts. The ore is mixed with limestone (caco₃) and coke (a form of carbon) and heated to very high temperatures. The main raw material is iron ore and this arrives in the uk. Iron is extracted from iron ore in a large container called a blast furnace. Concentration removes the water and other volatile impurities such as sulphur and carbonates. The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air) to remove water, decomposing carbonates into oxides, and converting sulfides. Iron is extracted in a large container called a blast furnace from its ore, hematite.
from y9mschemistry.blogspot.com
Iron(iii) oxide is reduced to molten iron when it reacts. Modern blast furnaces produce approximately 10,000 tonnes of iron per day. Concentration removes the water and other volatile impurities such as sulphur and carbonates. It’s a long process which begins with concentration through calcination roasting. The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air) to remove water, decomposing carbonates into oxides, and converting sulfides. Iron is extracted in a large container called a blast furnace from its ore, hematite. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the blast furnace. The main raw material is iron ore and this arrives in the uk. Fran scott explains iron extraction. Iron is extracted from iron ore in a large container called a blast furnace.
Year 9 Chemistry 2012 Extraction of iron from its ores
Describe How Iron Is Extracted From Its Ore Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the blast furnace. Iron(iii) oxide is reduced to molten iron when it reacts. Modern blast furnaces produce approximately 10,000 tonnes of iron per day. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the blast furnace. Iron is extracted from its ore (haematite, fe₂o₃) in a blast furnace. Fran scott explains iron extraction. Concentration removes the water and other volatile impurities such as sulphur and carbonates. It’s a long process which begins with concentration through calcination roasting. Iron is extracted in a large container called a blast furnace from its ore, hematite. The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air) to remove water, decomposing carbonates into oxides, and converting sulfides. How is iron extracted from its ore? The ore is mixed with limestone (caco₃) and coke (a form of carbon) and heated to very high temperatures. The main raw material is iron ore and this arrives in the uk. Iron is extracted from iron ore in a large container called a blast furnace. Iron is extracted from its ore using carbon in the form of coke.
From www.slideserve.com
PPT extraction of metals PowerPoint Presentation, free download ID4930166 Describe How Iron Is Extracted From Its Ore Concentration removes the water and other volatile impurities such as sulphur and carbonates. Iron is extracted in a large container called a blast furnace from its ore, hematite. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the blast furnace. The first step in the metallurgy of iron is usually roasting. Describe How Iron Is Extracted From Its Ore.
From www.youtube.com
Extraction Of Iron From Its Oxides YouTube Describe How Iron Is Extracted From Its Ore The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air) to remove water, decomposing carbonates into oxides, and converting sulfides. How is iron extracted from its ore? It’s a long process which begins with concentration through calcination roasting. The ore is mixed with limestone (caco₃) and coke (a form of carbon) and. Describe How Iron Is Extracted From Its Ore.
From www.chegg.com
Solved 2 Iron is extracted from its ore, hematite, in the Describe How Iron Is Extracted From Its Ore Iron is extracted from its ore (haematite, fe₂o₃) in a blast furnace. Concentration removes the water and other volatile impurities such as sulphur and carbonates. It’s a long process which begins with concentration through calcination roasting. How is iron extracted from its ore? Iron is extracted in a large container called a blast furnace from its ore, hematite. Redox reactions. Describe How Iron Is Extracted From Its Ore.
From www.w3schools.blog
Iron Extraction W3schools Describe How Iron Is Extracted From Its Ore Iron(iii) oxide is reduced to molten iron when it reacts. Iron is extracted in a large container called a blast furnace from its ore, hematite. How is iron extracted from its ore? Fran scott explains iron extraction. Iron is extracted from its ore (haematite, fe₂o₃) in a blast furnace. The first step in the metallurgy of iron is usually roasting. Describe How Iron Is Extracted From Its Ore.
From app.pandai.org
Extraction of Metals from its Ore Describe How Iron Is Extracted From Its Ore The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air) to remove water, decomposing carbonates into oxides, and converting sulfides. Modern blast furnaces produce approximately 10,000 tonnes of iron per day. It’s a long process which begins with concentration through calcination roasting. Iron is extracted in a large container called a blast. Describe How Iron Is Extracted From Its Ore.
From spmscience.blog.onlinetuition.com.my
5.4.3 Extraction of Metals from their Ores Using Coke SPM Science Describe How Iron Is Extracted From Its Ore How is iron extracted from its ore? The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air) to remove water, decomposing carbonates into oxides, and converting sulfides. Iron is extracted from its ore using carbon in the form of coke. Iron(iii) oxide is reduced to molten iron when it reacts. The ore. Describe How Iron Is Extracted From Its Ore.
From www.youtube.com
Extraction of Metals from Ores with basic concepts and Explanation with examples YouTube Describe How Iron Is Extracted From Its Ore Iron(iii) oxide is reduced to molten iron when it reacts. Iron is extracted from its ore using carbon in the form of coke. The main raw material is iron ore and this arrives in the uk. The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air) to remove water, decomposing carbonates into. Describe How Iron Is Extracted From Its Ore.
From www.onlinemathlearning.com
Industrial Chemistry IGCSE Chemistry (solutions, examples, worksheets, videos) Describe How Iron Is Extracted From Its Ore Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the blast furnace. The ore is mixed with limestone (caco₃) and coke (a form of carbon) and heated to very high temperatures. Iron(iii) oxide is reduced to molten iron when it reacts. How is iron extracted from its ore? Concentration removes the. Describe How Iron Is Extracted From Its Ore.
From classnotes.org.in
Extraction of Iron Class 12, General Principles and Processes of Isolation of Elements Describe How Iron Is Extracted From Its Ore Iron(iii) oxide is reduced to molten iron when it reacts. The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air) to remove water, decomposing carbonates into oxides, and converting sulfides. The main raw material is iron ore and this arrives in the uk. Iron is extracted from its ore using carbon in. Describe How Iron Is Extracted From Its Ore.
From www.askiitians.com
Extraction of Iron Study material for IIT JEE askIITians Describe How Iron Is Extracted From Its Ore Iron(iii) oxide is reduced to molten iron when it reacts. Modern blast furnaces produce approximately 10,000 tonnes of iron per day. How is iron extracted from its ore? Iron is extracted from its ore (haematite, fe₂o₃) in a blast furnace. It’s a long process which begins with concentration through calcination roasting. The first step in the metallurgy of iron is. Describe How Iron Is Extracted From Its Ore.
From www.slideserve.com
PPT Physical Properties of Metals PowerPoint Presentation ID5519685 Describe How Iron Is Extracted From Its Ore Fran scott explains iron extraction. Modern blast furnaces produce approximately 10,000 tonnes of iron per day. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the blast furnace. How is iron extracted from its ore? Iron is extracted from its ore using carbon in the form of coke. Iron is extracted. Describe How Iron Is Extracted From Its Ore.
From www.youtube.com
Extraction of Iron Making Iron in the Blast Furnace teachyourselfchemistry studyfromhome Describe How Iron Is Extracted From Its Ore Modern blast furnaces produce approximately 10,000 tonnes of iron per day. Iron is extracted from its ore using carbon in the form of coke. Fran scott explains iron extraction. Concentration removes the water and other volatile impurities such as sulphur and carbonates. The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air). Describe How Iron Is Extracted From Its Ore.
From www.youtube.com
Extraction of iron explained YouTube Describe How Iron Is Extracted From Its Ore The main raw material is iron ore and this arrives in the uk. Iron is extracted from its ore (haematite, fe₂o₃) in a blast furnace. It’s a long process which begins with concentration through calcination roasting. Iron(iii) oxide is reduced to molten iron when it reacts. How is iron extracted from its ore? Modern blast furnaces produce approximately 10,000 tonnes. Describe How Iron Is Extracted From Its Ore.
From byjus.com
How is iron extracted from hematite? Describe How Iron Is Extracted From Its Ore Concentration removes the water and other volatile impurities such as sulphur and carbonates. Iron is extracted from its ore (haematite, fe₂o₃) in a blast furnace. Iron(iii) oxide is reduced to molten iron when it reacts. Modern blast furnaces produce approximately 10,000 tonnes of iron per day. Iron is extracted from its ore using carbon in the form of coke. Fran. Describe How Iron Is Extracted From Its Ore.
From www.w3schools.blog
Iron Extraction W3schools Describe How Iron Is Extracted From Its Ore Iron is extracted from iron ore in a large container called a blast furnace. Concentration removes the water and other volatile impurities such as sulphur and carbonates. It’s a long process which begins with concentration through calcination roasting. The main raw material is iron ore and this arrives in the uk. Modern blast furnaces produce approximately 10,000 tonnes of iron. Describe How Iron Is Extracted From Its Ore.
From www.chegg.com
Solved 3. Iron is extracted from its ore using coke in a Describe How Iron Is Extracted From Its Ore Iron is extracted from its ore (haematite, fe₂o₃) in a blast furnace. Iron is extracted in a large container called a blast furnace from its ore, hematite. The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air) to remove water, decomposing carbonates into oxides, and converting sulfides. How is iron extracted from. Describe How Iron Is Extracted From Its Ore.
From en.ppt-online.org
Metals online presentation Describe How Iron Is Extracted From Its Ore Iron is extracted from its ore (haematite, fe₂o₃) in a blast furnace. Iron is extracted from its ore using carbon in the form of coke. Modern blast furnaces produce approximately 10,000 tonnes of iron per day. The main raw material is iron ore and this arrives in the uk. Iron is extracted from iron ore in a large container called. Describe How Iron Is Extracted From Its Ore.
From www.goodscience.com.au
Extraction of Metals Good Science Describe How Iron Is Extracted From Its Ore The ore is mixed with limestone (caco₃) and coke (a form of carbon) and heated to very high temperatures. How is iron extracted from its ore? Iron is extracted from iron ore in a large container called a blast furnace. Iron is extracted from its ore (haematite, fe₂o₃) in a blast furnace. The main raw material is iron ore and. Describe How Iron Is Extracted From Its Ore.
From www.sliderbase.com
Blast Furnace Presentation Chemistry Describe How Iron Is Extracted From Its Ore The main raw material is iron ore and this arrives in the uk. How is iron extracted from its ore? Modern blast furnaces produce approximately 10,000 tonnes of iron per day. Iron is extracted from its ore (haematite, fe₂o₃) in a blast furnace. Concentration removes the water and other volatile impurities such as sulphur and carbonates. Iron is extracted from. Describe How Iron Is Extracted From Its Ore.
From cityraven.com
😀 Extraction of iron from its oxides. mining extraction of iron from its oxide ore. 20190130 Describe How Iron Is Extracted From Its Ore How is iron extracted from its ore? Iron is extracted from iron ore in a large container called a blast furnace. Concentration removes the water and other volatile impurities such as sulphur and carbonates. Iron is extracted from its ore using carbon in the form of coke. The ore is mixed with limestone (caco₃) and coke (a form of carbon). Describe How Iron Is Extracted From Its Ore.
From www.revisechemistry.uk
Improving processes and products OCR Gateway C6 revisechemistry.uk Describe How Iron Is Extracted From Its Ore Iron is extracted from its ore using carbon in the form of coke. Iron is extracted in a large container called a blast furnace from its ore, hematite. Iron is extracted from iron ore in a large container called a blast furnace. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within. Describe How Iron Is Extracted From Its Ore.
From www.allthescience.org
How is Iron Refined from Ore? (with pictures) Describe How Iron Is Extracted From Its Ore How is iron extracted from its ore? The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air) to remove water, decomposing carbonates into oxides, and converting sulfides. The ore is mixed with limestone (caco₃) and coke (a form of carbon) and heated to very high temperatures. Fran scott explains iron extraction. Iron. Describe How Iron Is Extracted From Its Ore.
From mavink.com
Blast Furnace Labelled Diagram Describe How Iron Is Extracted From Its Ore The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air) to remove water, decomposing carbonates into oxides, and converting sulfides. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the blast furnace. Modern blast furnaces produce approximately 10,000 tonnes of iron per day.. Describe How Iron Is Extracted From Its Ore.
From askfilo.com
6 Iron is extracted from its ore, hematite, in the blast furnace.Describ.. Describe How Iron Is Extracted From Its Ore Iron(iii) oxide is reduced to molten iron when it reacts. Iron is extracted from its ore (haematite, fe₂o₃) in a blast furnace. Iron is extracted from iron ore in a large container called a blast furnace. Iron is extracted from its ore using carbon in the form of coke. The main raw material is iron ore and this arrives in. Describe How Iron Is Extracted From Its Ore.
From www.dreamstime.com
Iron Ore, Rocks from Which Metallic Iron Can Be Obtained, Iron Extracted from Describe How Iron Is Extracted From Its Ore The main raw material is iron ore and this arrives in the uk. Fran scott explains iron extraction. Iron is extracted from iron ore in a large container called a blast furnace. Concentration removes the water and other volatile impurities such as sulphur and carbonates. The ore is mixed with limestone (caco₃) and coke (a form of carbon) and heated. Describe How Iron Is Extracted From Its Ore.
From www.youtube.com
11 Iron extraction process (3rd year secondary) YouTube Describe How Iron Is Extracted From Its Ore Modern blast furnaces produce approximately 10,000 tonnes of iron per day. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the blast furnace. Concentration removes the water and other volatile impurities such as sulphur and carbonates. Iron is extracted from iron ore in a large container called a blast furnace. Iron. Describe How Iron Is Extracted From Its Ore.
From www.youtube.com
Iron extraction YouTube Describe How Iron Is Extracted From Its Ore Modern blast furnaces produce approximately 10,000 tonnes of iron per day. Iron(iii) oxide is reduced to molten iron when it reacts. The ore is mixed with limestone (caco₃) and coke (a form of carbon) and heated to very high temperatures. Iron is extracted from its ore using carbon in the form of coke. Iron is extracted from its ore (haematite,. Describe How Iron Is Extracted From Its Ore.
From www.youtube.com
Extraction of Iron From its Ore Class 12 Metallurgy Lecture by Shubhangi Sharma YouTube Describe How Iron Is Extracted From Its Ore How is iron extracted from its ore? It’s a long process which begins with concentration through calcination roasting. The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air) to remove water, decomposing carbonates into oxides, and converting sulfides. Redox reactions are involved in the extraction of metals from their ores, eg extracting. Describe How Iron Is Extracted From Its Ore.
From www.slideserve.com
PPT OBTAINING METALS PowerPoint Presentation, free download ID2068276 Describe How Iron Is Extracted From Its Ore Fran scott explains iron extraction. The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air) to remove water, decomposing carbonates into oxides, and converting sulfides. How is iron extracted from its ore? Iron is extracted from its ore (haematite, fe₂o₃) in a blast furnace. Iron(iii) oxide is reduced to molten iron when. Describe How Iron Is Extracted From Its Ore.
From www.quelpr.com
CSEC Chemistry Extraction of Metals (Iron) Describe How Iron Is Extracted From Its Ore How is iron extracted from its ore? Iron is extracted from its ore (haematite, fe₂o₃) in a blast furnace. Iron is extracted in a large container called a blast furnace from its ore, hematite. Redox reactions are involved in the extraction of metals from their ores, eg extracting iron by reduction within the blast furnace. It’s a long process which. Describe How Iron Is Extracted From Its Ore.
From www.bigstockphoto.com
Extraction Iron Ore Image & Photo (Free Trial) Bigstock Describe How Iron Is Extracted From Its Ore Iron is extracted from its ore (haematite, fe₂o₃) in a blast furnace. It’s a long process which begins with concentration through calcination roasting. Iron(iii) oxide is reduced to molten iron when it reacts. Concentration removes the water and other volatile impurities such as sulphur and carbonates. Iron is extracted from iron ore in a large container called a blast furnace.. Describe How Iron Is Extracted From Its Ore.
From y9mschemistry.blogspot.com
Year 9 Chemistry 2012 Extraction of iron from its ores Describe How Iron Is Extracted From Its Ore The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air) to remove water, decomposing carbonates into oxides, and converting sulfides. Concentration removes the water and other volatile impurities such as sulphur and carbonates. Iron(iii) oxide is reduced to molten iron when it reacts. Modern blast furnaces produce approximately 10,000 tonnes of iron. Describe How Iron Is Extracted From Its Ore.
From y9mschemistry.blogspot.com
Year 9 Chemistry 2012 Extraction of iron from its ores Describe How Iron Is Extracted From Its Ore Concentration removes the water and other volatile impurities such as sulphur and carbonates. The main raw material is iron ore and this arrives in the uk. Fran scott explains iron extraction. Iron is extracted from its ore using carbon in the form of coke. Iron(iii) oxide is reduced to molten iron when it reacts. The ore is mixed with limestone. Describe How Iron Is Extracted From Its Ore.
From www.slideserve.com
PPT Metal Ores PowerPoint Presentation ID5647941 Describe How Iron Is Extracted From Its Ore Iron is extracted from iron ore in a large container called a blast furnace. Iron is extracted from its ore using carbon in the form of coke. The ore is mixed with limestone (caco₃) and coke (a form of carbon) and heated to very high temperatures. Concentration removes the water and other volatile impurities such as sulphur and carbonates. Redox. Describe How Iron Is Extracted From Its Ore.
From www.slideserve.com
PPT Extraction of metals PowerPoint Presentation ID208800 Describe How Iron Is Extracted From Its Ore How is iron extracted from its ore? Modern blast furnaces produce approximately 10,000 tonnes of iron per day. Iron(iii) oxide is reduced to molten iron when it reacts. Iron is extracted from its ore using carbon in the form of coke. The first step in the metallurgy of iron is usually roasting the ore (heating the ore in air) to. Describe How Iron Is Extracted From Its Ore.