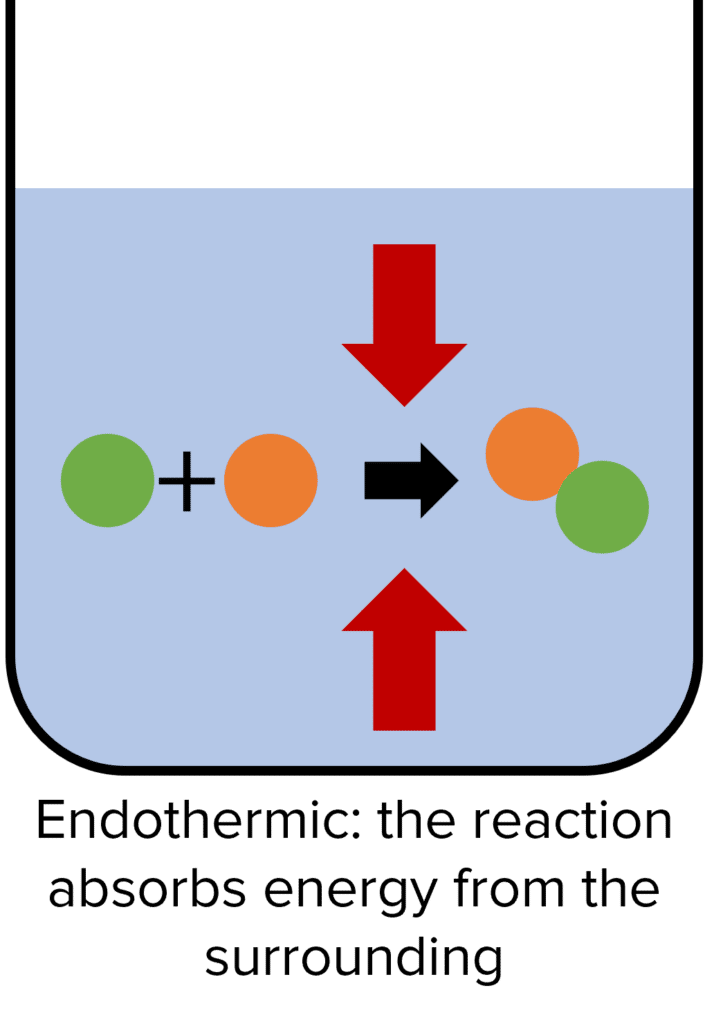
Endothermic Reaction Non Examples . Because heat is absorbed, endothermic reactions feel cold. Photosynthesis is a good example of an endothermic. When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction causes the surroundings to cool down. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. Some examples of endothermic reactions are: In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. For example, plants take in energy from the sun and convert carbon dioxide and water into glucose and oxygen. Conversely, an exothermic reaction is one.
from mmerevise.co.uk
Conversely, an exothermic reaction is one. For example, plants take in energy from the sun and convert carbon dioxide and water into glucose and oxygen. Photosynthesis is a good example of an endothermic. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Some examples of endothermic reactions are: In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. The reaction between ethanoic acid and sodium carbonate. When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of. An endothermic reaction causes the surroundings to cool down.
Endothermic and Exothermic Reactions Revision MME
Endothermic Reaction Non Examples Photosynthesis is a good example of an endothermic. Photosynthesis is a good example of an endothermic. An endothermic reaction causes the surroundings to cool down. Some examples of endothermic reactions are: An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of. The reaction between ethanoic acid and sodium carbonate. Because heat is absorbed, endothermic reactions feel cold. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Conversely, an exothermic reaction is one. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. For example, plants take in energy from the sun and convert carbon dioxide and water into glucose and oxygen. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Non Examples Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Photosynthesis is a good example of an endothermic. The reaction between ethanoic acid and sodium carbonate. Conversely, an exothermic reaction is one. In an endothermic process, the heat that a system absorbs is. Endothermic Reaction Non Examples.
From www.slideserve.com
PPT Endothermic and Exothermic Reactions PowerPoint Presentation, free download ID658850 Endothermic Reaction Non Examples When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Conversely, an exothermic reaction is one. An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. Photosynthesis is. Endothermic Reaction Non Examples.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Reaction Non Examples Conversely, an exothermic reaction is one. For example, plants take in energy from the sun and convert carbon dioxide and water into glucose and oxygen. An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. Photosynthesis is a good example of an endothermic. When 1mol 1 mol of calcium carbonate decomposes into 1 mol. Endothermic Reaction Non Examples.
From www.youtube.com
Endothermic Reaction and Exothermic Reaction YouTube Endothermic Reaction Non Examples Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction causes the surroundings to cool down. Photosynthesis is a good example of an endothermic. Because heat is absorbed, endothermic reactions feel cold. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings.. Endothermic Reaction Non Examples.
From facts.net
16 Intriguing Facts About Endothermic Endothermic Reaction Non Examples Some examples of endothermic reactions are: An endothermic reaction causes the surroundings to cool down. The reaction between ethanoic acid and sodium carbonate. When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of. An endothermic reaction occurs when energy is absorbed from the surroundings in the form of. Endothermic Reaction Non Examples.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition, Properties, Examples Endothermic Reaction Non Examples An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. An endothermic reaction causes the surroundings to cool down. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Photosynthesis is a good example of an endothermic. Because heat is absorbed, endothermic reactions feel cold. Conversely, an exothermic reaction is. Endothermic Reaction Non Examples.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Non Examples An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The reaction between ethanoic acid and sodium carbonate. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol. Endothermic Reaction Non Examples.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Non Examples The reaction between ethanoic acid and sodium carbonate. For example, plants take in energy from the sun and convert carbon dioxide and water into glucose and oxygen. Because heat is absorbed, endothermic reactions feel cold. Conversely, an exothermic reaction is one. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. When 1mol. Endothermic Reaction Non Examples.
From www.doubtnut.com
Distinguish between Endothermic reaction and Exothermic reactions. Endothermic Reaction Non Examples Conversely, an exothermic reaction is one. For example, plants take in energy from the sun and convert carbon dioxide and water into glucose and oxygen. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol. Endothermic Reaction Non Examples.
From www.diffzy.com
Exothermic vs. Endothermic Reactions What's the Difference (With Table) Endothermic Reaction Non Examples An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Photosynthesis is a good example of an endothermic. When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol. Endothermic Reaction Non Examples.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Non Examples Photosynthesis is a good example of an endothermic. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. An endothermic reaction causes the surroundings to cool down. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Some examples of endothermic reactions are: In an. Endothermic Reaction Non Examples.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Non Examples Some examples of endothermic reactions are: Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction causes the surroundings to cool down. For example, plants take in energy from the sun and convert carbon dioxide and water into glucose and oxygen. In an endothermic process, the heat that a system absorbs is thermal energy. Endothermic Reaction Non Examples.
From quizizz.com
Endothermic vs Exothermic Process Chemistry Quiz Quizizz Endothermic Reaction Non Examples Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. For example, plants take in energy from the sun and convert carbon dioxide and water into glucose and oxygen. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic reactions feel cold. Some examples of endothermic reactions are:. Endothermic Reaction Non Examples.
From testbook.com
Endothermic Reaction Learn Definition, Reagents, Formula here Endothermic Reaction Non Examples An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of. Because heat is absorbed, endothermic reactions feel cold. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the. Endothermic Reaction Non Examples.
From www.tes.com
Exothermic and Endothermic Reactions Teaching Resources Endothermic Reaction Non Examples The reaction between ethanoic acid and sodium carbonate. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Because heat is absorbed, endothermic reactions feel cold. Some examples of endothermic reactions are: Conversely, an exothermic reaction is one. When 1mol. Endothermic Reaction Non Examples.
From khatiar1955a.altervista.org
The Difference Between Endothermic and Exothermic Reactions Endothermic Reaction Non Examples When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of. The reaction between ethanoic acid and sodium carbonate. Because heat is absorbed, endothermic reactions feel cold. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. An endothermic reaction occurs when. Endothermic Reaction Non Examples.
From www.slideserve.com
PPT 6.02 ChemLive Exothermic and Exothermic Reactions PowerPoint Presentation ID1796730 Endothermic Reaction Non Examples An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. Because heat is absorbed, endothermic reactions feel cold. In an endothermic process, the heat that a system absorbs is thermal energy transfer. Endothermic Reaction Non Examples.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Non Examples Some examples of endothermic reactions are: An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. The reaction between ethanoic acid and sodium carbonate. Because heat is absorbed, endothermic reactions feel cold. When 1mol 1 mol of calcium carbonate decomposes. Endothermic Reaction Non Examples.
From 5differencebetween.com
5 Difference Between Endothermic and Exothermic Reaction Endothermic Vs Exothermic Endothermic Reaction Non Examples An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic reactions feel cold. An endothermic reaction causes the surroundings to cool down. The reaction between ethanoic acid and sodium carbonate. Endothermic and exothermic reactions are. Endothermic Reaction Non Examples.
From mungfali.com
Endothermic Diagram With Labels Endothermic Reaction Non Examples The reaction between ethanoic acid and sodium carbonate. Conversely, an exothermic reaction is one. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Photosynthesis is a good example of an endothermic. For example, plants take in energy from the sun and convert carbon dioxide and water into glucose and oxygen. An endothermic reaction is a chemical. Endothermic Reaction Non Examples.
From diseno.librogratis.info
Britain For Learners 2nd Ed Reading Libro Gratis Endothermic Reaction Non Examples For example, plants take in energy from the sun and convert carbon dioxide and water into glucose and oxygen. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Some examples of endothermic reactions are: An endothermic reaction causes. Endothermic Reaction Non Examples.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Endothermic Reaction Non Examples Some examples of endothermic reactions are: An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. The reaction between ethanoic acid and sodium carbonate. Because heat is absorbed, endothermic reactions feel cold. When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of.. Endothermic Reaction Non Examples.
From www.vrogue.co
Endothermic Reaction Definition Differences And Examp vrogue.co Endothermic Reaction Non Examples The reaction between ethanoic acid and sodium carbonate. When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Conversely, an exothermic reaction is one. Because heat is absorbed, endothermic reactions feel cold. For example, plants. Endothermic Reaction Non Examples.
From www.pinterest.co.uk
Endothermic and Exothermic Reactions Lab ⋆ Exothermic reaction, Teaching Endothermic Reaction Non Examples An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat.. Endothermic Reaction Non Examples.
From www.yaclass.in
Exothermic and Endothermic chemical changes — lesson. Science State Board, Class 8. Endothermic Reaction Non Examples Some examples of endothermic reactions are: Because heat is absorbed, endothermic reactions feel cold. Photosynthesis is a good example of an endothermic. When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of. For example, plants take in energy from the sun and convert carbon dioxide and water into. Endothermic Reaction Non Examples.
From www.youtube.com
Exothermic vs Endothermic Chemical Reactions YouTube Endothermic Reaction Non Examples An endothermic reaction causes the surroundings to cool down. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. Some examples of endothermic reactions are: Because heat. Endothermic Reaction Non Examples.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Resources Endothermic Reaction Non Examples When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of. The reaction between ethanoic acid and sodium carbonate. Conversely, an exothermic reaction is one. Because heat is absorbed, endothermic reactions feel cold. Some examples of endothermic reactions are: An endothermic reaction occurs when energy is absorbed from the. Endothermic Reaction Non Examples.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reaction Non Examples For example, plants take in energy from the sun and convert carbon dioxide and water into glucose and oxygen. The reaction between ethanoic acid and sodium carbonate. Photosynthesis is a good example of an endothermic. An endothermic reaction causes the surroundings to cool down. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat. Endothermic Reaction Non Examples.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction Non Examples An endothermic reaction causes the surroundings to cool down. An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. For example, plants take in energy from the sun and convert carbon dioxide and water into glucose and oxygen. The reaction between ethanoic acid and sodium carbonate. Photosynthesis is a good example of an endothermic.. Endothermic Reaction Non Examples.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? Endothermic Reaction Non Examples In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Photosynthesis is a good example of an endothermic. When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of. An endothermic reaction is a chemical reaction that absorbs thermal energy from its. Endothermic Reaction Non Examples.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction Non Examples Photosynthesis is a good example of an endothermic. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Conversely, an exothermic reaction is one. An endothermic reaction. Endothermic Reaction Non Examples.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction Non Examples Some examples of endothermic reactions are: An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. Because heat is absorbed, endothermic reactions feel cold. Conversely, an exothermic reaction is one. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. When 1mol. Endothermic Reaction Non Examples.
From www.vecteezy.com
Enthalpy Endothermic versus Endothermic biochemistry vector illustration graphic 21790110 Vector Endothermic Reaction Non Examples When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of. For example, plants take in energy from the sun and convert carbon dioxide and water into glucose and oxygen. An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. Conversely, an exothermic. Endothermic Reaction Non Examples.
From question.pandai.org
Endothermic and exothermic reactions Endothermic Reaction Non Examples The reaction between ethanoic acid and sodium carbonate. Photosynthesis is a good example of an endothermic. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Some examples of endothermic reactions are: Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. An endothermic reaction is a chemical reaction that. Endothermic Reaction Non Examples.
From eduinput.com
Difference Between Endothermic And Exothermic Reactions Endothermic Reaction Non Examples An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of. Endothermic and exothermic reactions are chemical reactions. Endothermic Reaction Non Examples.