Table Salt Mixed In Water Acts As Dash In Electrolysis . Mix up a different electrolyte solution by. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. In this part you will see how industrial chemists use electrolysis to produce useful chemicals. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. In a down's cell, the liquid sodium. Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. A down's cell is used for the electrolysis of molten sodium chloride.
from www.teachoo.com
Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. Mix up a different electrolyte solution by. In this part you will see how industrial chemists use electrolysis to produce useful chemicals. A down's cell is used for the electrolysis of molten sodium chloride. In a down's cell, the liquid sodium. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series.
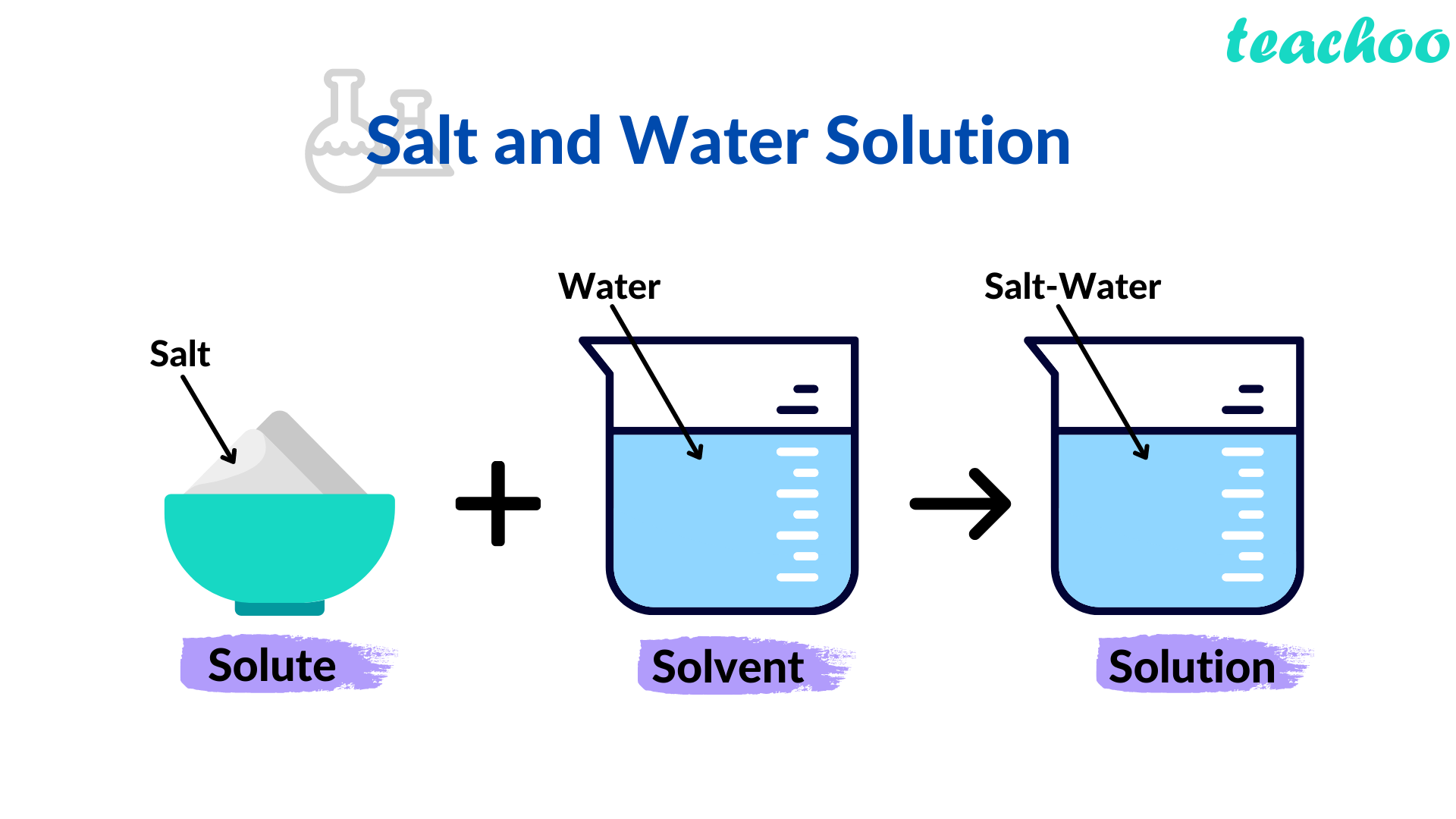
Solution Definition, Types, Properties Chemistry Teachoo
Table Salt Mixed In Water Acts As Dash In Electrolysis In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. A down's cell is used for the electrolysis of molten sodium chloride. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. In a down's cell, the liquid sodium. Mix up a different electrolyte solution by. Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. In this part you will see how industrial chemists use electrolysis to produce useful chemicals.
From www.youtube.com
table salt dissolves in water YouTube Table Salt Mixed In Water Acts As Dash In Electrolysis In this part you will see how industrial chemists use electrolysis to produce useful chemicals. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. Using a mixed salt system means. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.alamy.com
Saline solution salt liquid water Stock Vector Images Alamy Table Salt Mixed In Water Acts As Dash In Electrolysis In a down's cell, the liquid sodium. Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. Mix up a different electrolyte solution by. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. Electrolysis of an aqueous solution of table salt (nacl,. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.slideserve.com
PPT Creating Compounds PowerPoint Presentation, free download ID1980578 Table Salt Mixed In Water Acts As Dash In Electrolysis In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. In a down's cell, the liquid sodium. Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. In this part you will see how industrial chemists use electrolysis to produce useful chemicals. I. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.colourbox.com
The process of dissociation of table salt (sodium chloride) in water. Stock vector Colourbox Table Salt Mixed In Water Acts As Dash In Electrolysis A down's cell is used for the electrolysis of molten sodium chloride. In this part you will see how industrial chemists use electrolysis to produce useful chemicals. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. Mix up a different electrolyte solution by. In a down's cell, the liquid sodium. I. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.dreamstime.com
Sodium Chloride (rock Salt, Halite, Table Salt), Crystal Structure Stock Illustration Table Salt Mixed In Water Acts As Dash In Electrolysis In a down's cell, the liquid sodium. Mix up a different electrolyte solution by. A down's cell is used for the electrolysis of molten sodium chloride. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. In this part you will see how industrial chemists use electrolysis to produce useful chemicals. I. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From group.chem.iastate.edu
Conductivity Meter Holme Research Group Iowa State University Table Salt Mixed In Water Acts As Dash In Electrolysis Mix up a different electrolyte solution by. In a down's cell, the liquid sodium. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. In this explainer, we will learn how. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.thoughtco.com
Chemical Composition of Table Salt Table Salt Mixed In Water Acts As Dash In Electrolysis In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. In a down's cell, the liquid sodium. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.teachoo.com
Solution Definition, Types, Properties Chemistry Teachoo Table Salt Mixed In Water Acts As Dash In Electrolysis I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. In this part you will see how industrial chemists use electrolysis to produce useful chemicals. Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. In this explainer, we will learn how to. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From klublr.com
Química De Mistura Homogênea Table Salt Mixed In Water Acts As Dash In Electrolysis Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. Mix up a different electrolyte solution by. In a down's cell, the liquid sodium. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From brainly.in
what is salt bridge plz describe ? give its two function Brainly.in Table Salt Mixed In Water Acts As Dash In Electrolysis In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Mix up a different electrolyte solution by. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. Using a mixed salt system means there is a possibility of. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.semanticscholar.org
Table 2 from Effect of Methanol on Carbonate Equilibrium and Calcite Solubility in a Gas Table Salt Mixed In Water Acts As Dash In Electrolysis In this part you will see how industrial chemists use electrolysis to produce useful chemicals. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. In a down's cell, the liquid sodium. A. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.chegg.com
Solved A 4,500 litre tank contains 2,500 litres of brine. Table Salt Mixed In Water Acts As Dash In Electrolysis Mix up a different electrolyte solution by. Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. In this part you will see how industrial chemists use electrolysis to produce useful chemicals. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Electrolysis. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.dreamstime.com
Sodium Chloride or Rock Salt, Halite, Table Salt, Chemical Structure. Stock Vector Table Salt Mixed In Water Acts As Dash In Electrolysis Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. In a down's cell, the liquid sodium. A down's cell is used for the electrolysis of molten sodium chloride. Mix up a different. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.semanticscholar.org
Figure 1 from Recovery of dyes and salts from highly concentrated (dye and salt) mixed water Table Salt Mixed In Water Acts As Dash In Electrolysis Mix up a different electrolyte solution by. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water,. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.researchgate.net
Dielectric properties of table salt solutions as a function of... Download Scientific Diagram Table Salt Mixed In Water Acts As Dash In Electrolysis Mix up a different electrolyte solution by. In a down's cell, the liquid sodium. Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. In this part you will see how industrial chemists. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From courses.lumenlearning.com
Concentration Units Boundless Chemistry Table Salt Mixed In Water Acts As Dash In Electrolysis Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. Mix up a different electrolyte. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From exoialiwb.blob.core.windows.net
Mix Water And Table Salt at Mathew Long blog Table Salt Mixed In Water Acts As Dash In Electrolysis Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Mix up a different electrolyte solution by. In a down's cell, the liquid sodium. A down's cell is used for the electrolysis of. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From gbu-taganskij.ru
Sodium Chloride Table Salt Chemical Structure Vector Image, 40 OFF Table Salt Mixed In Water Acts As Dash In Electrolysis Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. In this part you will see how industrial chemists use electrolysis to produce useful chemicals. A down's cell is used for. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From twobirdsfourhands.com
Chemical Formula For Iodized Table Salt Two Birds Home Table Salt Mixed In Water Acts As Dash In Electrolysis Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. Mix up a different electrolyte solution by. In this part you will see how industrial chemists use electrolysis to produce useful chemicals. In a down's cell, the liquid sodium. A down's cell is used for the electrolysis of molten sodium chloride. Electrolysis of an. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.coursehero.com
[Solved] Table salt contains 39.33 g of sodium per 100 g of salt. The U.S.... Course Hero Table Salt Mixed In Water Acts As Dash In Electrolysis In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. In this part you will see how industrial chemists use electrolysis to produce useful chemicals. Mix up a different electrolyte solution by. A down's cell is used for the electrolysis of molten sodium chloride. Using a mixed salt system. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.mdpi.com
JMSE Free FullText Microparticles in Table Salt Levels and Chemical Composition of the Table Salt Mixed In Water Acts As Dash In Electrolysis In a down's cell, the liquid sodium. Mix up a different electrolyte solution by. A down's cell is used for the electrolysis of molten sodium chloride. Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From present5.com
Anode Cathode Electrolysis Finished 8 Table Salt Mixed In Water Acts As Dash In Electrolysis Mix up a different electrolyte solution by. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. A down's cell is used for the electrolysis of molten sodium chloride. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. Using a. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? in 2020 Chemical changes Table Salt Mixed In Water Acts As Dash In Electrolysis In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. A down's cell is used for the electrolysis of molten sodium chloride. In this part you will see how industrial chemists use electrolysis to produce useful chemicals. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.numerade.com
SOLVED How could you distinguish sodium chloride (table salt) from sodium iodide (a poison Table Salt Mixed In Water Acts As Dash In Electrolysis A down's cell is used for the electrolysis of molten sodium chloride. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. In a down's cell, the liquid sodium. In this part you will see how industrial chemists use electrolysis to produce useful chemicals. Using a mixed salt system. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From study.com
The AcidBase Properties of Water Video & Lesson Transcript Table Salt Mixed In Water Acts As Dash In Electrolysis In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. In this part you will see how industrial chemists use electrolysis to produce useful chemicals. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. I want to decompose $\ce{h}$ ion. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From shaunmwilliams.com
Chapter 11 Presentation Table Salt Mixed In Water Acts As Dash In Electrolysis In a down's cell, the liquid sodium. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. A down's cell is used for the electrolysis of molten sodium chloride. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. I want. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.saubhaya.com
Table Salt Chemical Makeup Saubhaya Makeup Table Salt Mixed In Water Acts As Dash In Electrolysis A down's cell is used for the electrolysis of molten sodium chloride. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Electrolysis of an aqueous solution of. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From studylib.net
LAB The Synthesis of Table Salt Table Salt Mixed In Water Acts As Dash In Electrolysis A down's cell is used for the electrolysis of molten sodium chloride. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. In a down's cell, the liquid sodium. Using a. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.chegg.com
Solved 2 The dissolution of table salt in water is a Table Salt Mixed In Water Acts As Dash In Electrolysis Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. In this part you will see how industrial chemists use electrolysis to produce useful chemicals. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. In a down's cell, the liquid sodium. A. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.shutterstock.com
Salt formula Images, Stock Photos & Vectors Shutterstock Table Salt Mixed In Water Acts As Dash In Electrolysis Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. Mix up a different electrolyte solution by. A down's cell is used for the electrolysis of molten sodium chloride. In this part you will see how industrial chemists use electrolysis to produce useful chemicals. In a down's cell, the liquid sodium. Using. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.sciencephoto.com
Dissociation of table salt in water, illustration Stock Image F027/1895 Science Photo Library Table Salt Mixed In Water Acts As Dash In Electrolysis In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Mix up a different electrolyte solution by. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. Using a mixed salt system means there is a possibility of. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From www.baristahustle.com
TWC 0.02 How Does Water Dissolve Mineral Salts? Barista Hustle Table Salt Mixed In Water Acts As Dash In Electrolysis In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. I want to decompose $\ce{h}$. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From sciencing.com
What Happens When Salt Is Added to Water? Sciencing Table Salt Mixed In Water Acts As Dash In Electrolysis I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. In this explainer, we will. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From chem.libretexts.org
3.6 Changes in Matter Physical and Chemical Changes Chemistry LibreTexts Table Salt Mixed In Water Acts As Dash In Electrolysis Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. I want to decompose $\ce{h}$ ion and $\ce{o}$ ion by putting carbon electrode in the water, but it look so slow. Using a mixed salt system means there is a possibility of competition between different electrolytic reactions. A down's cell is used. Table Salt Mixed In Water Acts As Dash In Electrolysis.
From studylib.net
Properties of table salt Table Salt Mixed In Water Acts As Dash In Electrolysis In this part you will see how industrial chemists use electrolysis to produce useful chemicals. Mix up a different electrolyte solution by. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. A down's cell is used for the electrolysis of molten sodium chloride. In a down's cell, the liquid sodium. I. Table Salt Mixed In Water Acts As Dash In Electrolysis.