Aluminum Acetate Dissociation Equation . reactions in aqueous solutions. additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base). Because acetic acid is a weak acid, its ka is measurable. ch 3co − 2 (aq) + h 2o(l) ⇌ ch 3co 2h(aq) + oh − (aq) kb = kw / ka. when nacl dissolves in water, the ions separate and go their own way in solution; The ions are now written with their. in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide.
from studygripewater.z21.web.core.windows.net
because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base). when nacl dissolves in water, the ions separate and go their own way in solution; in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. reactions in aqueous solutions. The ions are now written with their. ch 3co − 2 (aq) + h 2o(l) ⇌ ch 3co 2h(aq) + oh − (aq) kb = kw / ka. Because acetic acid is a weak acid, its ka is measurable.
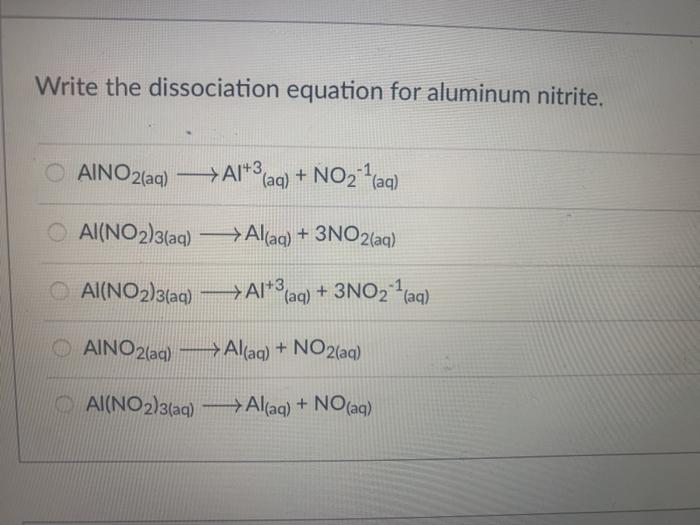
How To Write Dissociation Equations
Aluminum Acetate Dissociation Equation however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. reactions in aqueous solutions. Because acetic acid is a weak acid, its ka is measurable. in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base). when nacl dissolves in water, the ions separate and go their own way in solution; The ions are now written with their. however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. ch 3co − 2 (aq) + h 2o(l) ⇌ ch 3co 2h(aq) + oh − (aq) kb = kw / ka.
From www.numerade.com
Complex the equation for the dissociation equilibrium of acetic acid in Aluminum Acetate Dissociation Equation The ions are now written with their. additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. reactions in aqueous solutions. when nacl dissolves in water, the ions separate. Aluminum Acetate Dissociation Equation.
From dxodgqmah.blob.core.windows.net
Chemical Formula For Aluminum And Oxygen at Patrick Moyer blog Aluminum Acetate Dissociation Equation ch 3co − 2 (aq) + h 2o(l) ⇌ ch 3co 2h(aq) + oh − (aq) kb = kw / ka. reactions in aqueous solutions. additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. Because acetic acid is a weak acid, its ka is measurable. The ions are. Aluminum Acetate Dissociation Equation.
From www.numerade.com
SOLVED what are the products for the reaction of aluminium acetate and Aluminum Acetate Dissociation Equation additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is. Aluminum Acetate Dissociation Equation.
From www.chegg.com
Solved 4. Consider The Chemical Named Aluminum Acetate A Aluminum Acetate Dissociation Equation because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base). Because acetic acid is a weak acid, its ka is measurable. in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? ch 3co − 2 (aq) + h 2o(l). Aluminum Acetate Dissociation Equation.
From www.youtube.com
Equation for Al(OH)3 + H2O (Aluminum Hydroxide + Water) YouTube Aluminum Acetate Dissociation Equation however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. ch 3co − 2. Aluminum Acetate Dissociation Equation.
From aluminumgenjin.blogspot.com
Aluminum Formula For Aluminum Acetate Aluminum Acetate Dissociation Equation in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? when nacl dissolves in water, the ions separate and go their own way in solution; because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base). additionally, aluminum acetate. Aluminum Acetate Dissociation Equation.
From www.tessshebaylo.com
Dissociation Of Acetic Acid Equation Tessshebaylo Aluminum Acetate Dissociation Equation because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base). when nacl dissolves in water, the ions separate and go their own way in solution; however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide.. Aluminum Acetate Dissociation Equation.
From www.chegg.com
Solved A solution contains 1.31×10−2M aluminum acetate and Aluminum Acetate Dissociation Equation additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. Because acetic acid is a weak acid, its ka is measurable. reactions in aqueous solutions. when nacl dissolves in water, the ions separate and go their own way in solution; ch 3co − 2 (aq) + h 2o(l). Aluminum Acetate Dissociation Equation.
From exoaasvxy.blob.core.windows.net
What Is Aluminum Acetate Formula at Maria Marchan blog Aluminum Acetate Dissociation Equation however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. ch 3co − 2 (aq) + h 2o(l) ⇌ ch 3co 2h(aq) + oh − (aq) kb = kw / ka. The ions are now written with their. when nacl dissolves in water, the ions separate and go. Aluminum Acetate Dissociation Equation.
From www.youtube.com
Biochemistry 3.2 Dissociation of acids YouTube Aluminum Acetate Dissociation Equation when nacl dissolves in water, the ions separate and go their own way in solution; because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base). ch 3co − 2 (aq) + h 2o(l) ⇌ ch 3co 2h(aq) + oh − (aq) kb = kw. Aluminum Acetate Dissociation Equation.
From www.youtube.com
How to write chemical formula of Aluminium acetate YouTube Aluminum Acetate Dissociation Equation in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? Because acetic acid is a weak acid, its ka is measurable. however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. reactions in aqueous solutions. The ions are now written with their. . Aluminum Acetate Dissociation Equation.
From www.researchgate.net
Energy of dissociation and electron separation in a negative aluminum Aluminum Acetate Dissociation Equation ch 3co − 2 (aq) + h 2o(l) ⇌ ch 3co 2h(aq) + oh − (aq) kb = kw / ka. Because acetic acid is a weak acid, its ka is measurable. reactions in aqueous solutions. because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a. Aluminum Acetate Dissociation Equation.
From www.coursehero.com
[Solved] what are aluminum acetate cation and anion and formula? what Aluminum Acetate Dissociation Equation ch 3co − 2 (aq) + h 2o(l) ⇌ ch 3co 2h(aq) + oh − (aq) kb = kw / ka. Because acetic acid is a weak acid, its ka is measurable. reactions in aqueous solutions. when nacl dissolves in water, the ions separate and go their own way in solution; however, the acetate ion, the. Aluminum Acetate Dissociation Equation.
From www.numerade.com
SOLVEDWhen \mathrm{H}_{2} \mathrm{S} is passed through an aqueous Aluminum Acetate Dissociation Equation Because acetic acid is a weak acid, its ka is measurable. reactions in aqueous solutions. however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. when nacl dissolves in water, the ions separate and go their own way in solution; in a 0.23 mol/l aluminum sulfate solution,. Aluminum Acetate Dissociation Equation.
From studygripewater.z21.web.core.windows.net
How To Write Dissociation Equations Aluminum Acetate Dissociation Equation reactions in aqueous solutions. additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. The ions are now written with their. however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. ch 3co − 2 (aq) + h 2o(l). Aluminum Acetate Dissociation Equation.
From www.chegg.com
Solved Dissociation One Aluminum Sulfide Aluminum Sulfide Aluminum Acetate Dissociation Equation because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base). Because acetic acid is a weak acid, its ka is measurable. in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? The ions are now written with their. additionally,. Aluminum Acetate Dissociation Equation.
From www.tessshebaylo.com
Dissociation Of Acetic Acid In Water Net Ionic Equation Tessshebaylo Aluminum Acetate Dissociation Equation The ions are now written with their. additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. reactions in aqueous solutions. when nacl dissolves in water, the ions separate and go their own way in solution; however, the acetate ion, the conjugate base of acetic acid, reacts with. Aluminum Acetate Dissociation Equation.
From www.numerade.com
SOLVED Write a balanced equation to show the reaction of aqueous Aluminum Acetate Dissociation Equation ch 3co − 2 (aq) + h 2o(l) ⇌ ch 3co 2h(aq) + oh − (aq) kb = kw / ka. because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base). reactions in aqueous solutions. when nacl dissolves in water, the ions separate. Aluminum Acetate Dissociation Equation.
From aluminumgenjin.blogspot.com
Aluminum Formula For Aluminum Acetate Aluminum Acetate Dissociation Equation in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? ch 3co − 2 (aq) + h 2o(l) ⇌ ch 3co 2h(aq) + oh − (aq) kb = kw / ka. because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a. Aluminum Acetate Dissociation Equation.
From aluminumgenjin.blogspot.com
Aluminum Formula For Aluminum Acetate Aluminum Acetate Dissociation Equation however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. Because acetic acid is a weak acid, its ka is measurable. ch 3co − 2 (aq) + h 2o(l) ⇌ ch 3co 2h(aq) + oh − (aq) kb = kw / ka. because acetic acid is a weak. Aluminum Acetate Dissociation Equation.
From www.gelest.com
ALUMINUM ACETATE, DIBASIC, tech Gelest, Inc. Aluminum Acetate Dissociation Equation The ions are now written with their. because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base). however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. when nacl dissolves in water, the ions separate. Aluminum Acetate Dissociation Equation.
From www.numerade.com
SOLVED 'Answer the following stoichiometryrelated questions 12 Aluminum Acetate Dissociation Equation additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? when nacl dissolves in. Aluminum Acetate Dissociation Equation.
From www.coursehero.com
[Solved] For each chemical listed, write the dissociation / ionization Aluminum Acetate Dissociation Equation additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. Because acetic acid is a weak acid, its ka is measurable. however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. in a 0.23 mol/l aluminum sulfate solution, what is. Aluminum Acetate Dissociation Equation.
From studygripewater.z21.web.core.windows.net
How To Write Dissociation Equations Aluminum Acetate Dissociation Equation because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base). however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion?. Aluminum Acetate Dissociation Equation.
From www.youtube.com
How to Write the Formula for Aluminum acetate YouTube Aluminum Acetate Dissociation Equation because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base). Because acetic acid is a weak acid, its ka is measurable. The ions are now written with their. when nacl dissolves in water, the ions separate and go their own way in solution; however,. Aluminum Acetate Dissociation Equation.
From www.shutterstock.com
Aluminium Acetate Handwritten Chemical Formula On Stock Illustration Aluminum Acetate Dissociation Equation in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? reactions in aqueous solutions. however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. ch 3co − 2 (aq) + h 2o(l) ⇌ ch 3co 2h(aq) + oh − (aq) kb =. Aluminum Acetate Dissociation Equation.
From www.coursehero.com
[Solved] Write dissociation reactions for the following soluble Aluminum Acetate Dissociation Equation The ions are now written with their. additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. when nacl dissolves in water, the ions separate and go their own way. Aluminum Acetate Dissociation Equation.
From www.numerade.com
SOLVED The dissociation equation of Acetic acid (CH3COOH) is shown Aluminum Acetate Dissociation Equation however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. when nacl dissolves in water, the ions separate and go their own way in solution; because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base).. Aluminum Acetate Dissociation Equation.
From testbook.com
Aluminium Acetate Formula Structure, Properties & Uses. Aluminum Acetate Dissociation Equation when nacl dissolves in water, the ions separate and go their own way in solution; Because acetic acid is a weak acid, its ka is measurable. additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. because acetic acid is a weak acid, its k a is measurable and. Aluminum Acetate Dissociation Equation.
From www.numerade.com
SOLVED Write dissociation reactions of the following ionic compounds Aluminum Acetate Dissociation Equation however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide. Because acetic acid is a weak acid, its ka is measurable. in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum. Aluminum Acetate Dissociation Equation.
From www.tessshebaylo.com
Dissociation Of Acetic Acid Equation Tessshebaylo Aluminum Acetate Dissociation Equation additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base). however, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the. Aluminum Acetate Dissociation Equation.
From www.slideserve.com
PPT Chapter 13 Ions in Aqueous Solutions and Colligative Properties Aluminum Acetate Dissociation Equation reactions in aqueous solutions. in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? Because acetic acid is a weak acid, its ka is measurable. The ions are now written with their. additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous. because. Aluminum Acetate Dissociation Equation.
From www.numerade.com
SOLVED The dissociation equation of Acetic acid (CH3COOH) is shown Aluminum Acetate Dissociation Equation reactions in aqueous solutions. because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base). when nacl dissolves in water, the ions separate and go their own way in solution; in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each. Aluminum Acetate Dissociation Equation.
From www.researchgate.net
Thermal of aluminum sulfate and aluminum acetate Aluminum Acetate Dissociation Equation The ions are now written with their. when nacl dissolves in water, the ions separate and go their own way in solution; Because acetic acid is a weak acid, its ka is measurable. ch 3co − 2 (aq) + h 2o(l) ⇌ ch 3co 2h(aq) + oh − (aq) kb = kw / ka. because acetic acid. Aluminum Acetate Dissociation Equation.
From www.chegg.com
Solved 7. Write a balanced equation for the dissociation Aluminum Acetate Dissociation Equation because acetic acid is a weak acid, its k a is measurable and k b > 0 (acetate ion is a weak base). in a 0.23 mol/l aluminum sulfate solution, what is the molar concentration of each ion? when nacl dissolves in water, the ions separate and go their own way in solution; Because acetic acid is. Aluminum Acetate Dissociation Equation.