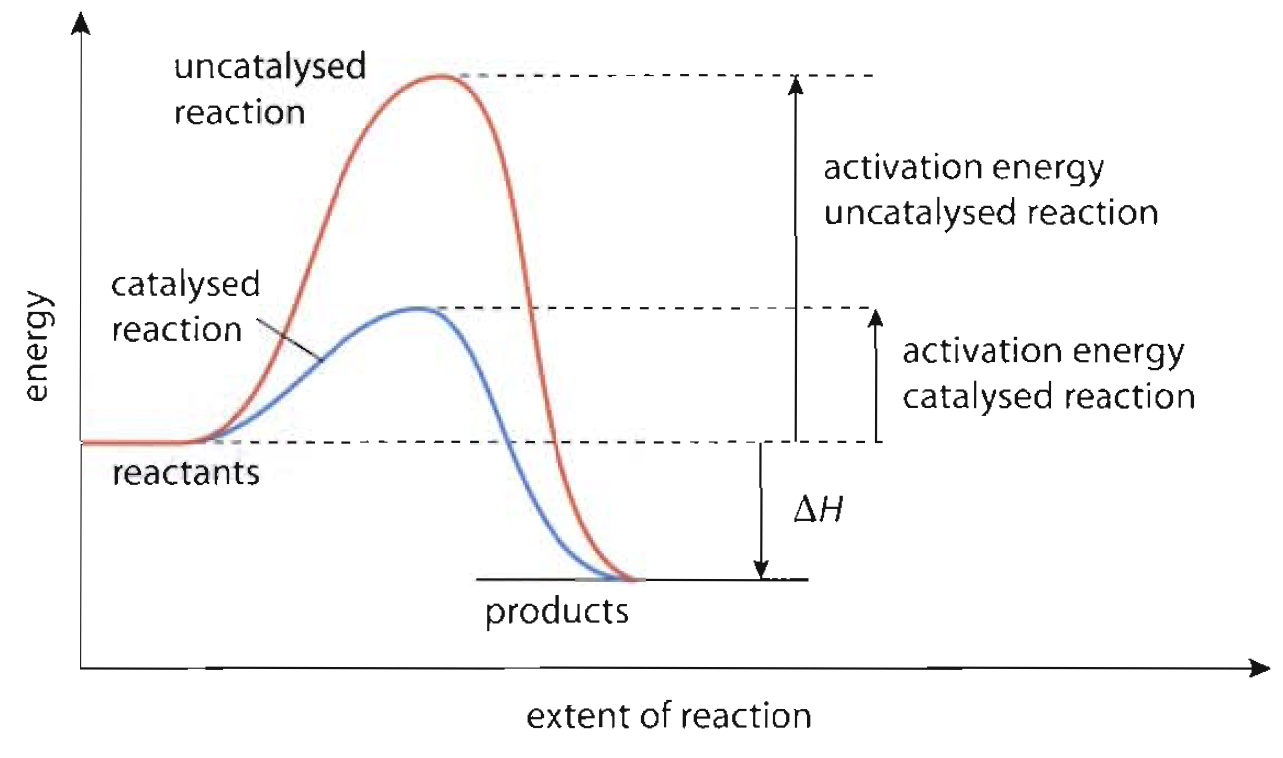
Catalyst Change A Reaction . Is not chemically changed or used up at the end of the. A catalyst is a substance that speeds up a chemical reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. this page explains how adding a catalyst affects the rate of a reaction. When the reaction has finished, you would have. a catalyst lowers the activation energy of a reaction, increasing its rate. among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a. It is not consumed by the process. a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. A catalyst is a substance that can help the reactants in a chemical reaction react with each other. catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible. In chemistry and biology, a catalyst is. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a. a catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
from exoddixpp.blob.core.windows.net
homogeneous catalysis refers to reactions in which the catalyst is in solution with at least one of the reactants whereas heterogeneous catalysis. a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. It is not consumed by the process. plots of the normalized kpis are then used to demonstrate the best catalyst using two case studies: Increases the rate of a reaction. a catalyst lowers the activation energy of a reaction, increasing its rate. A catalyst is a substance that can help the reactants in a chemical reaction react with each other. Increases the rate of a reaction. this page explains how adding a catalyst affects the rate of a reaction. a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction.
Define Catalyst Science Term at Michael Moorehead blog
Catalyst Change A Reaction When the reaction has finished, you would have. Increases the rate of a reaction. The catalyst is not used up or chemically changed. herein, we proposed an entropy variation strategy to develop a dynamic cuzn−co/heos catalyst, in which the non. plots of the normalized kpis are then used to demonstrate the best catalyst using two case studies: a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. It is not consumed by the process. a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. catalysts, by their definition, is a substance that increases the rate of a chemical reaction without changing themselves. because of the complexity of different electrocatalytic reactions, studying catalytic mechanisms requires exploring. When the reaction has finished, you would have. a catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a. this page explains how adding a catalyst affects the rate of a reaction. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy. a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Change A Reaction catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the. this page explains how adding a catalyst affects the rate of a reaction. among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a. because of. Catalyst Change A Reaction.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Change A Reaction Increases the rate of a reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible. a catalyst. Catalyst Change A Reaction.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalyst Change A Reaction Increases the rate of a reaction. because of the complexity of different electrocatalytic reactions, studying catalytic mechanisms requires exploring. plots of the normalized kpis are then used to demonstrate the best catalyst using two case studies: catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible. catalyst, in chemistry, any substance that. Catalyst Change A Reaction.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Catalyst Change A Reaction catalysts, by their definition, is a substance that increases the rate of a chemical reaction without changing themselves. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a.. Catalyst Change A Reaction.
From www.nagwa.com
Question Video Understanding How Catalysts Affect the Rates and the Catalyst Change A Reaction a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. homogeneous catalysis refers to reactions in which the catalyst is in solution with at least one of the reactants whereas heterogeneous catalysis. catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition. Catalyst Change A Reaction.
From chemistry.stackexchange.com
physical chemistry Which diagram shows the effect of catalysis on Catalyst Change A Reaction because of the complexity of different electrocatalytic reactions, studying catalytic mechanisms requires exploring. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Increases the rate of a reaction. catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed. Catalyst Change A Reaction.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst Change A Reaction a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. this page explains how adding a catalyst affects the rate of a reaction. catalysts function by allowing the reaction to take place through an alternative mechanism that requires a. When the reaction has finished, you would have.. Catalyst Change A Reaction.
From study.com
Effect of Catalysts on Rates of Reaction Lesson Catalyst Change A Reaction It assumes familiarity with basic concepts. a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. this page explains how adding a catalyst affects the rate of a reaction. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy.. Catalyst Change A Reaction.
From exoddixpp.blob.core.windows.net
Define Catalyst Science Term at Michael Moorehead blog Catalyst Change A Reaction When the reaction has finished, you would have. Increases the rate of a reaction. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. herein, we proposed an entropy variation strategy to develop a dynamic cuzn−co/heos catalyst, in which the non. a catalyst is another substance than reactants products added to. Catalyst Change A Reaction.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalyst Change A Reaction Is not chemically changed or used up at the end of the. A catalyst is a substance that can help the reactants in a chemical reaction react with each other. A catalyst is a substance that speeds up a chemical reaction. The catalyst is not used up or chemically changed. It assumes familiarity with basic concepts. a catalyst lowers. Catalyst Change A Reaction.
From exoqpbamm.blob.core.windows.net
A Catalyst Increases The Rate Of A Reaction By Providing A Path That at Catalyst Change A Reaction among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a. plots of the normalized kpis are then used to demonstrate the best catalyst using two case studies: catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. this page. Catalyst Change A Reaction.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst Change A Reaction catalysts, by their definition, is a substance that increases the rate of a chemical reaction without changing themselves. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. herein, we proposed an entropy variation strategy to develop a dynamic cuzn−co/heos catalyst, in which the non. catalysts, pressure, temperature. Catalyst Change A Reaction.
From schematicdiagramyakuza.z13.web.core.windows.net
Catalyst Diagram Chemistry Catalyst Change A Reaction It assumes familiarity with basic concepts. catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible. A catalyst is a substance that can help the reactants in a chemical reaction react with each other. The catalyst is not used up or chemically changed. catalyst, in chemistry, any substance that increases the rate of a. Catalyst Change A Reaction.
From www.linstitute.net
IB DP Chemistry HL复习笔记6.1.8 Energy Profiles & Catalysis翰林国际教育 Catalyst Change A Reaction The catalyst is not used up or chemically changed. Is not chemically changed or used up at the end of the. homogeneous catalysis refers to reactions in which the catalyst is in solution with at least one of the reactants whereas heterogeneous catalysis. among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence. Catalyst Change A Reaction.
From www.slideserve.com
PPT §10.5 Catalytic reaction PowerPoint Presentation, free download Catalyst Change A Reaction because of the complexity of different electrocatalytic reactions, studying catalytic mechanisms requires exploring. catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of a reversible. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy. catalysts affect the rate of a chemical reaction by altering its mechanism to. Catalyst Change A Reaction.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Catalyst Change A Reaction Is not chemically changed or used up at the end of the. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy. among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a. homogeneous catalysis refers to reactions in which the catalyst is. Catalyst Change A Reaction.
From exorsjyuc.blob.core.windows.net
Catalyst Reaction Meaning at Jarred Mikula blog Catalyst Change A Reaction plots of the normalized kpis are then used to demonstrate the best catalyst using two case studies: a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. In chemistry and biology, a catalyst is. catalysts, pressure, temperature & concentration of solutions can affect equilibrium position. Catalyst Change A Reaction.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalyst Change A Reaction catalysts function by allowing the reaction to take place through an alternative mechanism that requires a. catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy. Increases the rate of a reaction. among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a.. Catalyst Change A Reaction.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalyst Change A Reaction When the reaction has finished, you would have. In chemistry and biology, a catalyst is. a catalyst lowers the activation energy of a reaction, increasing its rate. A catalyst is a substance that can help the reactants in a chemical reaction react with each other. homogeneous catalysis refers to reactions in which the catalyst is in solution with. Catalyst Change A Reaction.
From 2012books.lardbucket.org
Catalysis Catalyst Change A Reaction among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a. herein, we proposed an entropy variation strategy to develop a dynamic cuzn−co/heos catalyst, in which the non. catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the. this page explains. Catalyst Change A Reaction.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.2.6 Practical Effect of Catalysts on Catalyst Change A Reaction a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. a catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a. In chemistry and biology, a catalyst is. Is a substance that increases the. Catalyst Change A Reaction.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalyst Change A Reaction The catalyst is not used up or chemically changed. a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the. herein, we proposed an. Catalyst Change A Reaction.
From ar.inspiredpencil.com
Catalyst Chemical Reaction Catalyst Change A Reaction It assumes familiarity with basic concepts. Is not chemically changed or used up at the end of the. this page explains how adding a catalyst affects the rate of a reaction. Increases the rate of a reaction. a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the. Catalyst Change A Reaction.
From www.youtube.com
How does a CATALYST work ? YouTube Catalyst Change A Reaction In chemistry and biology, a catalyst is. a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. a catalyst lowers the activation energy of a reaction, increasing its rate. plots of the normalized kpis are then used to demonstrate the best catalyst using two case. Catalyst Change A Reaction.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of Catalyst Change A Reaction this page explains how adding a catalyst affects the rate of a reaction. Increases the rate of a reaction. Is not chemically changed or used up at the end of the. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. because of the complexity of different electrocatalytic reactions,. Catalyst Change A Reaction.
From socratic.org
Why does a catalyst cause a reaction to speed up? Socratic Catalyst Change A Reaction a catalyst lowers the activation energy of a reaction, increasing its rate. among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a. Is not chemically changed or used up at the end of the. When the reaction has finished, you would have. homogeneous catalysis refers to reactions in. Catalyst Change A Reaction.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Catalyst Change A Reaction Increases the rate of a reaction. homogeneous catalysis refers to reactions in which the catalyst is in solution with at least one of the reactants whereas heterogeneous catalysis. plots of the normalized kpis are then used to demonstrate the best catalyst using two case studies: catalysts, pressure, temperature & concentration of solutions can affect equilibrium position of. Catalyst Change A Reaction.
From proper-cooking.info
Catalyst Chemical Reaction Catalyst Change A Reaction catalysts, by their definition, is a substance that increases the rate of a chemical reaction without changing themselves. catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the. a catalyst is a substance that is used to speed up a chemical reaction but it is. Catalyst Change A Reaction.
From www.youtube.com
Practice Identifying Catalysts and Intermediates YouTube Catalyst Change A Reaction Increases the rate of a reaction. Increases the rate of a reaction. When the reaction has finished, you would have. catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the. catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in. Catalyst Change A Reaction.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Change A Reaction catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy. plots of the normalized kpis are then used to demonstrate the best catalyst using two case studies: among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a. Increases the rate of a. Catalyst Change A Reaction.
From bianca-well-hooper.blogspot.com
Example of Combination Reaction in Which Catalyst Is Used Catalyst Change A Reaction catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. When the reaction has finished, you would have. Increases the rate of a reaction. catalysts affect the rate of a chemical reaction by altering. Catalyst Change A Reaction.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Change A Reaction a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. Increases the rate of a reaction. among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a. It is not consumed by the process. Increases the rate of. Catalyst Change A Reaction.
From schoolbag.info
A catalyst speeds up a reaction by providing the reactants with an Catalyst Change A Reaction catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy. Is not chemically changed or used up at the end of the. A catalyst is a substance that speeds up a chemical reaction. It assumes familiarity with basic concepts. A catalyst is a substance that can help the reactants in a chemical reaction. Catalyst Change A Reaction.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Change A Reaction In chemistry and biology, a catalyst is. homogeneous catalysis refers to reactions in which the catalyst is in solution with at least one of the reactants whereas heterogeneous catalysis. The catalyst is not used up or chemically changed. among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a. . Catalyst Change A Reaction.
From www.youtube.com
6.2.6 / 6.2.7 Describe the effect of a catalyst on a chemical reaction Catalyst Change A Reaction It assumes familiarity with basic concepts. a catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. catalysts affect the rate of a chemical reaction by. Catalyst Change A Reaction.