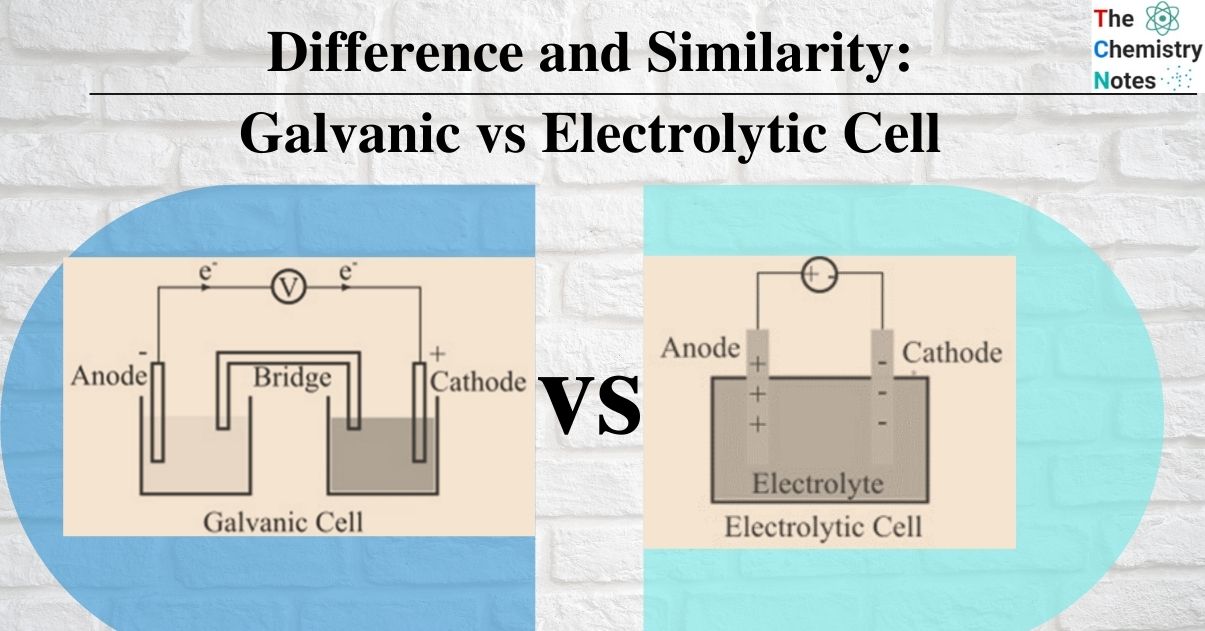
Electrochemical And Electrolytic Cell Comparison . Electric energy is converted to chemical energy in. The major distinction between an. describe the basic components of electrochemical cells. an electrolytic cell does the opposite, converting electrical energy into chemical energy. from the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces. it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Galvanic cells generate electrical energy through spontaneous redox. difference between electrolytic and electrochemical cell basic principle: electrochemical cells convert chemical energy to electrical energy, while electrolytic cells use electrical energy to drive. the voltaic cell and the electrolytic cell are both electrochemical cells which are at the heart of electrochemistry. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. What is a voltaic cell? in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to note their individual. These cells are called electrolytic cells.
from thechemistrynotes.com
What is a voltaic cell? the two primary types of electrochemical cells are. the main differences are outlined below: In an electrolytic cell, however, the opposite process, called electrolysis, occurs: describe the basic components of electrochemical cells. Galvanic cells generate electrical energy through spontaneous redox. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. List some of the characteristics, applications. voltaic cells and electrolytic cells are the two forms of electrochemical cells. the most significant difference between an electrolytic cell and an electrochemical cell is that an.
Difference and Similarity Galvanic vs Electrolytic Cell
Electrochemical And Electrolytic Cell Comparison it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. the most significant difference between an electrolytic cell and an electrochemical cell is that an. the main differences are outlined below: ultimately, while both electrochemical and electrolytic cells involve the movement of electrons and. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. the main difference between the two is their function. from the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces. the voltaic cell and the electrolytic cell are both electrochemical cells which are at the heart of electrochemistry. Galvanic cells (also known as voltaic cells) 2. in electrochemical cells, a spontaneous reaction occurs. an electric cell made of two different metals in contact with an electrolyte, produces a voltage across the metals. The major distinction between an. List some of the characteristics, applications. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: the two primary types of electrochemical cells are. These cells are called electrolytic cells.
From www.differencebetween.com
What is the Difference Between Voltaic Cell and Electrolytic Cell Electrochemical And Electrolytic Cell Comparison an electrolytic cell does the opposite, converting electrical energy into chemical energy. an electric cell made of two different metals in contact with an electrolyte, produces a voltage across the metals. The major distinction between an. from the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces. describe the basic. Electrochemical And Electrolytic Cell Comparison.
From study.com
Voltaic vs. Electrolytic Cell Similarities, Differences & Uses Electrochemical And Electrolytic Cell Comparison the main differences are outlined below: What is a voltaic cell? describe the basic components of electrochemical cells. ultimately, while both electrochemical and electrolytic cells involve the movement of electrons and. the two primary types of electrochemical cells are. the voltaic cell and the electrolytic cell are both electrochemical cells which are at the heart. Electrochemical And Electrolytic Cell Comparison.
From www.slideserve.com
PPT ELECTROCHEMICAL CELLS PowerPoint Presentation, free download ID Electrochemical And Electrolytic Cell Comparison the most significant difference between an electrolytic cell and an electrochemical cell is that an. ultimately, while both electrochemical and electrolytic cells involve the movement of electrons and. Galvanic cells generate electrical energy through spontaneous redox. components of electrochemical cells. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: the voltaic cell and the. Electrochemical And Electrolytic Cell Comparison.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii Electrochemical And Electrolytic Cell Comparison when an electric current is passed through the cell, ions migrate towards the electrodes, where they. Learn about electrolytic cells with diagrams and faqs. List some of the characteristics, applications. electrochemical cells convert chemical energy to electrical energy, while electrolytic cells use electrical energy to drive. An electrochemical cell splits the oxidant and reductant in a manner that. Electrochemical And Electrolytic Cell Comparison.
From www.dynamicscience.com.au
redox reactions electrolysis The difference between electrochemical Electrochemical And Electrolytic Cell Comparison the voltaic cell and the electrolytic cell are both electrochemical cells which are at the heart of electrochemistry. voltaic cells and electrolytic cells are the two forms of electrochemical cells. describe the basic components of electrochemical cells. components of electrochemical cells. it is possible to construct a cell that does work on a chemical system. Electrochemical And Electrolytic Cell Comparison.
From www.coursehero.com
[Solved] differentiate voltaic cell and electrolytic cell. tabulate Electrochemical And Electrolytic Cell Comparison the main difference between the two is their function. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. in electrochemical cells, a spontaneous reaction occurs. Learn about electrolytic cells with diagrams and faqs. the voltaic cell and the electrolytic cell are both electrochemical cells which are at the. Electrochemical And Electrolytic Cell Comparison.
From www.youtube.com
Comparison of Electrolytic and Galvanic Cells Chy9 Chp7 Electrochemical And Electrolytic Cell Comparison In an electrolytic cell, however, the opposite process, called electrolysis, occurs: in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to note their individual. These cells are called electrolytic cells. ultimately, while both electrochemical and electrolytic cells involve the movement of electrons and. describe the basic components of electrochemical. Electrochemical And Electrolytic Cell Comparison.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrochemical And Electrolytic Cell Comparison What is a voltaic cell? when an electric current is passed through the cell, ions migrate towards the electrodes, where they. in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to note their individual. Galvanic cells generate electrical energy through spontaneous redox. describe the basic components of electrochemical cells.. Electrochemical And Electrolytic Cell Comparison.
From fixcarbattery.blogspot.com
Recondition Car battery Magnesium copper water battery Electrochemical And Electrolytic Cell Comparison List some of the characteristics, applications. What is a voltaic cell? Galvanic cells generate electrical energy through spontaneous redox. the main differences are outlined below: the two primary types of electrochemical cells are. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. from the above differences between galvanic and. Electrochemical And Electrolytic Cell Comparison.
From worksheetlistid.z21.web.core.windows.net
Lesson 5 Electrolytic Cells Electrochemical And Electrolytic Cell Comparison an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. electrochemical cells convert chemical energy to electrical energy, while electrolytic cells use electrical energy to drive. What is a voltaic cell? An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. in electrochemical. Electrochemical And Electrolytic Cell Comparison.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell Electrochemical And Electrolytic Cell Comparison electrochemical cells convert chemical energy to electrical energy, while electrolytic cells use electrical energy to drive. The major distinction between an. What is a voltaic cell? when an electric current is passed through the cell, ions migrate towards the electrodes, where they. voltaic cells and electrolytic cells are the two forms of electrochemical cells. Learn about electrolytic. Electrochemical And Electrolytic Cell Comparison.
From www.youtube.com
Comparison between the electrolytic cell and chemical cell YouTube Electrochemical And Electrolytic Cell Comparison an electrolytic cell does the opposite, converting electrical energy into chemical energy. ultimately, while both electrochemical and electrolytic cells involve the movement of electrons and. These cells are called electrolytic cells. describe the basic components of electrochemical cells. in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to. Electrochemical And Electrolytic Cell Comparison.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Electrochemical And Electrolytic Cell Comparison electrochemical cells convert chemical energy to electrical energy, while electrolytic cells use electrical energy to drive. These cells are called electrolytic cells. the voltaic cell and the electrolytic cell are both electrochemical cells which are at the heart of electrochemistry. Differences between a galvanic cell and an electrolytic cell. components of electrochemical cells. Learn about electrolytic cells. Electrochemical And Electrolytic Cell Comparison.
From www.youtube.com
Electrochemical Cell vs Electrolytic Cell Electrochemistry YouTube Electrochemical And Electrolytic Cell Comparison describe the basic components of electrochemical cells. voltaic cells and electrolytic cells are the two forms of electrochemical cells. an electrolytic cell does the opposite, converting electrical energy into chemical energy. Learn about electrolytic cells with diagrams and faqs. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. These. Electrochemical And Electrolytic Cell Comparison.
From fixlibrarymarkbladgr.z13.web.core.windows.net
Cathode Charge In Electrolytic Cell Electrochemical And Electrolytic Cell Comparison difference between electrolytic and electrochemical cell basic principle: it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. voltaic cells and electrolytic cells are the two forms of electrochemical cells. Learn about electrolytic cells with diagrams and faqs. Galvanic cells generate electrical energy through spontaneous. Electrochemical And Electrolytic Cell Comparison.
From mavink.com
Differentiate Between Electrochemical Cell And Electrolytic Cell Electrochemical And Electrolytic Cell Comparison an electrolytic cell does the opposite, converting electrical energy into chemical energy. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. an electric cell made of two different metals in contact with an electrolyte, produces a voltage across the metals. The major distinction between an. voltaic cells and electrolytic. Electrochemical And Electrolytic Cell Comparison.
From www.youtube.com
9.2 Comparison of electrochemical cells (SL) YouTube Electrochemical And Electrolytic Cell Comparison These cells are called electrolytic cells. from the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces. the two primary types of electrochemical cells are. in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to note their individual. an electrolytic cell does. Electrochemical And Electrolytic Cell Comparison.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Electrochemical And Electrolytic Cell Comparison Galvanic cells (also known as voltaic cells) 2. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. an electrolytic cell does the opposite, converting electrical energy into chemical energy. Galvanic cells generate electrical energy through spontaneous redox. an apparatus that is used to generate electricity from a spontaneous redox reaction. Electrochemical And Electrolytic Cell Comparison.
From lavelle.chem.ucla.edu
Galvanic vs Voltaic Cells CHEMISTRY COMMUNITY Electrochemical And Electrolytic Cell Comparison Galvanic cells (also known as voltaic cells) 2. when an electric current is passed through the cell, ions migrate towards the electrodes, where they. Electric energy is converted to chemical energy in. an electric cell made of two different metals in contact with an electrolyte, produces a voltage across the metals. it is possible to construct a. Electrochemical And Electrolytic Cell Comparison.
From www.meritnation.com
Differentiate between electrolytic and electrochemical cell with Electrochemical And Electrolytic Cell Comparison Learn about electrolytic cells with diagrams and faqs. Galvanic cells generate electrical energy through spontaneous redox. ultimately, while both electrochemical and electrolytic cells involve the movement of electrons and. electrochemical cells convert chemical energy to electrical energy, while electrolytic cells use electrical energy to drive. an electric cell made of two different metals in contact with an. Electrochemical And Electrolytic Cell Comparison.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses Electrochemical And Electrolytic Cell Comparison an electric cell made of two different metals in contact with an electrolyte, produces a voltage across the metals. the voltaic cell and the electrolytic cell are both electrochemical cells which are at the heart of electrochemistry. voltaic cells and electrolytic cells are the two forms of electrochemical cells. difference between electrolytic and electrochemical cell basic. Electrochemical And Electrolytic Cell Comparison.
From www.slideserve.com
PPT ELECTROCHEMICAL CELLS PowerPoint Presentation, free download ID Electrochemical And Electrolytic Cell Comparison Galvanic cells (also known as voltaic cells) 2. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. describe the basic components of electrochemical cells. the most significant difference between an electrolytic cell and an electrochemical cell is that an. voltaic cells and electrolytic cells are the two forms of. Electrochemical And Electrolytic Cell Comparison.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Electrochemical And Electrolytic Cell Comparison voltaic cells and electrolytic cells are the two forms of electrochemical cells. ultimately, while both electrochemical and electrolytic cells involve the movement of electrons and. the main difference between the two is their function. Learn about electrolytic cells with diagrams and faqs. components of electrochemical cells. Differences between a galvanic cell and an electrolytic cell. . Electrochemical And Electrolytic Cell Comparison.
From chemistry.stackexchange.com
electrochemistry Linking an electrochemical cell to an electrolytic Electrochemical And Electrolytic Cell Comparison the most significant difference between an electrolytic cell and an electrochemical cell is that an. Galvanic cells (also known as voltaic cells) 2. voltaic cells and electrolytic cells are the two forms of electrochemical cells. when an electric current is passed through the cell, ions migrate towards the electrodes, where they. List some of the characteristics, applications.. Electrochemical And Electrolytic Cell Comparison.
From pubs.rsc.org
In search of widening the electrochemical window of solid electrolytes Electrochemical And Electrolytic Cell Comparison in electrochemical cells, a spontaneous reaction occurs. the two primary types of electrochemical cells are. Differences between a galvanic cell and an electrolytic cell. describe the basic components of electrochemical cells. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. An electrochemical cell splits the oxidant and reductant. Electrochemical And Electrolytic Cell Comparison.
From worksheetlistid.z21.web.core.windows.net
Lesson 5 Electrolytic Cells Electrochemical And Electrolytic Cell Comparison the main differences are outlined below: voltaic cells and electrolytic cells are the two forms of electrochemical cells. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. an apparatus that is used. Electrochemical And Electrolytic Cell Comparison.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Electrochemical And Electrolytic Cell Comparison Galvanic cells (also known as voltaic cells) 2. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: components of electrochemical cells. in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to note their individual. it is possible to construct a cell that does work on a chemical. Electrochemical And Electrolytic Cell Comparison.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells Electrochemical And Electrolytic Cell Comparison in electrochemical cells, a spontaneous reaction occurs. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: voltaic cells and electrolytic cells are the two forms of electrochemical cells. electrochemical cells convert chemical energy to electrical energy, while electrolytic cells use electrical energy to drive. the two primary types of electrochemical cells are. an. Electrochemical And Electrolytic Cell Comparison.
From sciencestruck.com
Similarities and Differences Between Voltaic Cells and Electrolytic Cells Electrochemical And Electrolytic Cell Comparison What is a voltaic cell? Differences between a galvanic cell and an electrolytic cell. describe the basic components of electrochemical cells. the voltaic cell and the electrolytic cell are both electrochemical cells which are at the heart of electrochemistry. the main differences are outlined below: an electric cell made of two different metals in contact with. Electrochemical And Electrolytic Cell Comparison.
From slidetodoc.com
Electrochemistry Spontaneity of Redox Reactions 21 1 Electrochemistry Electrochemical And Electrolytic Cell Comparison List some of the characteristics, applications. Electric energy is converted to chemical energy in. when an electric current is passed through the cell, ions migrate towards the electrodes, where they. the main differences are outlined below: What is a voltaic cell? In an electrolytic cell, however, the opposite process, called electrolysis, occurs: voltaic cells and electrolytic cells. Electrochemical And Electrolytic Cell Comparison.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrochemical And Electrolytic Cell Comparison These cells are called electrolytic cells. Electric energy is converted to chemical energy in. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. in electrochemical cells, a spontaneous reaction occurs. ultimately, while both electrochemical and electrolytic cells involve the movement of electrons and. Galvanic cells generate electrical energy through spontaneous. Electrochemical And Electrolytic Cell Comparison.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrochemical And Electrolytic Cell Comparison an electrolytic cell does the opposite, converting electrical energy into chemical energy. electrochemical cells convert chemical energy to electrical energy, while electrolytic cells use electrical energy to drive. difference between electrolytic and electrochemical cell basic principle: Differences between a galvanic cell and an electrolytic cell. in order to understand the difference between an electrochemical cell and. Electrochemical And Electrolytic Cell Comparison.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell Electrochemical And Electrolytic Cell Comparison the voltaic cell and the electrolytic cell are both electrochemical cells which are at the heart of electrochemistry. Galvanic cells (also known as voltaic cells) 2. the main difference between the two is their function. the most significant difference between an electrolytic cell and an electrochemical cell is that an. List some of the characteristics, applications. . Electrochemical And Electrolytic Cell Comparison.
From www.thoughtco.com
Electrochemical Cell Definition Electrochemical And Electrolytic Cell Comparison in electrochemical cells, a spontaneous reaction occurs. Differences between a galvanic cell and an electrolytic cell. List some of the characteristics, applications. Galvanic cells (also known as voltaic cells) 2. the two primary types of electrochemical cells are. describe the basic components of electrochemical cells. Electric energy is converted to chemical energy in. What is a voltaic. Electrochemical And Electrolytic Cell Comparison.
From www.vrogue.co
Chapter 3 Electrochemistry Galvanic Cell Part 1 Youtu vrogue.co Electrochemical And Electrolytic Cell Comparison ultimately, while both electrochemical and electrolytic cells involve the movement of electrons and. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. it is possible to construct a cell that does work on a chemical system by driving an electric current through the system. components of electrochemical cells. List. Electrochemical And Electrolytic Cell Comparison.